
Environmental Services (EVS) teams play a pivotal role in safeguarding the healthcare environment. Their responsibilities include maintaining cleanliness, preventing the spread of infections, and ensuring patient safety. To set EVS teams up for success, it is essential to equip them with effective and efficient cleaning and disinfecting products.
The Advantages of Ready to Use (RTU) Products
Ready to Use (RTU) products offer several advantages over traditional dilutable disinfectants. While RTU disinfectants may appear more expensive at first glance, evaluating the true cost and impact reveals their value:
- Efficacy: Dilutable disinfectants require proper mixing to achieve the correct concentration. Unfortunately, human error and dilution machine inaccuracies are inherent risks. If the in-use concentration falls below the recommended level, the disinfectant may not effectively eliminate dangerous pathogens. This oversight could leave vulnerable patients at risk.
- Contamination: Even when the dilution liquid is correctly prepared, reusable microfiber cloths pose contamination risks. A study found that after laundering, 93% of reusable microfiber towels still contained harmful bacteria1. Cross-contamination on surfaces becomes a concern, jeopardizing patient safety.
- Hidden Costs: Dilutable disinfectants demand additional labor and resources. The time spent preparing solutions and training staff on dilution ratios adds to operational costs. In contrast, RTU products streamline the process, reducing labor requirements and minimizing hidden expenses.
The Impact on Patient Safety
Choosing the right disinfectants directly affects patient safety. By opting for RTU products, healthcare facilities can:
- Minimize Errors: RTU products, such as pre-moistened wipes, eliminate the risk of incorrect dilution, ensuring consistent and effective disinfection. With RTU disinfection products, healthcare facilities can have confidence that the EVS team is applying the right amount of disinfectant, for the right contact time, every time.
- Reduce Contamination: When RTU products are being used according to directions for use, the risk of cross-contamination or variation in concentration is eliminated since there is no need for mixing, dilution machines, or laundering. Reusable cloths can harbor bacteria and other pathogens, but RTU products help to mitigate this risk.
- Enhance Efficiency and Compliance: When using RTU disinfectants, no mixing and dilution training is required, helping staff save time and effort. Further, with the ability to use RTU products in an instant, staff can efficiently and effectively work through compliant room turnover, so patients get the critical care they need as soon as possible. RTU solutions save time, allowing EVS teams to focus on patient care.
In the pursuit of patient safety, investing in Ready-to-Use products is a strategic decision. Products like Clorox Healthcare® Bleach Germicidal Disinfectants not only help to protect patients but also streamline operations and reduce hidden costs.
Remember, patient safety is everyone’s responsibility. Let’s continue to prioritize it in our healthcare facilities by equipping our teams with the tools they need to succeed, from the ER to the OR and beyond.
References
1. Sifuentes LY, Gerba CP, Weart I, Engelbrecht K, Koenig DW. Microbial contamination of hospital reusable cleaning towels. Am J Infect Control. 2013 Oct;41(10):912–5. doi: 10.1016/jajic.2013.01.015. Epub 2013 Mar 22. PMID: 23523522

Many of us have fallen victim to clickbait or flashy headlines designed to grab our attention. But those headlines don't always lead to meaningful, informed research. So how do we separate the new and useful information from the misleading work? As a research scientist with over a decade of research experience, I’ve got a few tips to help you evaluate research studies like a seasoned pro.

Tips for finding quality research studies
- Check the Sources – Journal and Funding
- Peer-reviewed journals are considered most reliable because they are often carefully evaluated by experts in the field; but even then, discretion should be applied. Since peer review is considered the gold standard, some journals may attempt to mimic peer-reviewed articles but instead publish without rigorous peer review or for a fee. For example, BioRxiv is an open-access online publication platform for articles that have not undergone peer review or editing before posting. To ensure credibility, use trusted online databases like PubMed, Academic Search Premiere or AcademicOneFile to access and assess publications.
- Additionally, research studies can be funded by various sources, including industry sponsors. You can find funding sources in the article. While it's essential to remain mindful of potential biases, it's equally important not to disregard a study solely based on its funding source. Industry-funded studies play a significant role in the research community, particularly in areas with limited funding. Ultimately, the quality of a study should be determined by the strength of its evidence and methodology, rather than its funding source alone.
- Dig into the Methodology
- Consider not just what was found, but how it was found. For instance, imagine a study concludes that a new disinfectant is better at killing pathogens compared to a standard disinfectant. If the methods show that the study did not use an EPA-registered disinfectant or failed to consider important factors like starting contamination levels, proper cleaning techniques, or adherence to the disinfectant’s directions for use and contact times, you may begin to question the results. Plus, if a study lacks a clear and detailed methods section, that's a reason to raise an eyebrow!
- Keep It Real — Assess Relevance and Applicability in the Real World
- When assessing a study's relevance and applicability, consider whether its findings can be generalized to broader contexts. For example, if a study shows the efficacy of a dietary intervention for managing a specific health condition in controlled settings with carefully monitored diets, evaluating whether the intervention can be generalized to real-world scenarios may involve considering factors like cultural dietary preferences, socioeconomic status and food accessibility.
- Similarly, if the study was conducted in a laboratory setting, consider how closely the test environment mimics real-world scenarios. While laboratory conditions may not fully replicate the complexities of the real world, they can provide valuable insights and allow for rigorous testing of hypotheses under controlled conditions.
- Scrutinize the Results and Conclusions
- Be cautious of studies that exaggerate their findings or draw conclusions unsupported by the data. Look for studies with well-supported and cautiously interpreted results. When a new study gains media attention, it can be common for headlines to sensationalize the findings to create the impression of a groundbreaking discovery. However, most study results contribute to existing knowledge by adding another piece to the puzzle.
- To assess the credibility of a study, consider if its findings align with prior research. Multiple studies reporting similar results strengthen the evidence base. For laboratory-based research, ensure that experiments were performed in duplicate or multiple times to confirm the consistency of results and minimize the impact of experimental errors on the conclusions.
Navigating the worldwide web of information with confidence and clarity
All in all, whether you're an Infection Preventionist (IP) staying in the loop on the latest research, a seasoned researcher or a cleaning professional, knowing how to assess the credibility of a study is a critical skill. In a world overflowing with information, it's easy to get lost in flashy headlines and misleading claims. Fortunately, there’s a lot of great and valuable content out there — you just need to know where to find it and how to critically analyze it. And once you do, keep applying discretion. It's vital that we approach clickbait and sensationalized headlines with a discerning mindset. So, let's continue to question, analyze and scrutinize research studies to ensure we're making informed decisions.

With almost 60% of in-house facility managers identifying training as a top issue, we know that training is crucial in the cleaning industry.1 However, it is very time consuming to create and execute on your own, and sometimes supplemental training is needed. Fortunately, there are many training options now available, but how do you know if they are worth your time? The good news is there is an established model of assessing training outcomes that can help!
In this blog, I will introduce you to the Kirkpatrick Model by covering 1) What it is, 2) How it can be used, and, 3) some of the results from the HealthyClean Certificate Program as an example. 2
Let's get started...
What is the Kirkpatrick model?
The Kirkpatrick model is a method of measuring learning effectiveness. It was developed in the 1950s by Don Kirkpatrick as part of his Ph.D. work at the University of Wisconsin. Since then, the model has become well established in the instructional design industry, becoming the leading model to assess if learning outcomes have been achieved .3
The Kirkpatrick model uses four levels to assess learning. The levels begin with the basics (did participants enjoy the course?) and move to bigger impacts of the training (did the training positively impact the business?). The table below outlines the four levels, what questions each seeks to answer, and the data used to answer each question.
| Level | Question | Data used |
| Participants Reaction | Did the participants enjoy the training and feel it was relevant to their job? | End of course survey that asks the learner what they felt about the training |
| Learning | Did the participants learn the content as was intended? | End of course evaluations (also called tests, quizzes, or assessments) |
| Behavior Change | Did the participants apply what they learned on the job? | Observation or survey after returning to work |
| Business Results | Were business outcomes achieved because of the training? | Pre-established Key Performance Indicators (KPIs). e.g., less complaints, turnover, or injuries |
Using the Kirkpatrick model
In an ideal world, all levels of the Kirkpatrick model would be assessed for all courses. In my experience, however, outcomes from any level are hard to come by. Time, money, resources, and knowing how to measure and what to ask for are barriers to understanding the true effectiveness of training programs. But, in an era where training is of utmost importance, and where everyone is stretched for time, assessing training for effectiveness and impact is one of the best things we can do to make sure we are using our time wisely.
So how can you use the information above to determine if a course is worth your time? Here are three suggestions:
- Review the training's learning objectives and ensure they meet your staff's learning needs.
- It’s a hard stop if the learning objectives don’t set out to train on what your employee's needs.
- The training should also test the learner on each learning objective to ensure they learned the information. A good rule of thumb is one question per learning objective, but there may be more.
- Ask the course developer for the outcomes of the training.
- Use the table above to help explain what questions you are looking to answer and understand the data they provide.
- Only move forward if you like the results, or if you are satisfied with the information that was provided.
- Pilot test with a few employees and do your own assessment.
- Participant Reaction: Ask your pilot group these questions:
- Did they like the training?
- Did they learn valuable information that will help them in their job?
- Did they think the training was a good use of their time?
- Learning: Ask your pilot group what they learned and see if it matches the stated learning objectives
- Behavior Change: Monitor to see if those in your pilot group apply the learnings from the training.
- Business Results: Keep track of KPIs that are important to your organization to see if there are larger business impacts after the pilot team took the training.
- Participant Reaction: Ask your pilot group these questions:
HealthyClean Results
If you’ve been following the CloroxPro journey into the world of high-quality education and training, you know that we now offer two ANSI-National Accreditation Board courses, and that to obtain those accreditations, we follow a global standard for measuring training outcomes.4
But what you may not know is that the ASTM standard requires certificate issuers to use an established model like Kirkpatrick's to monitor Participant Reaction and Learning. The goal of doing so is to drive continuous improvement, which is one of the ways that the training delivers quality over time.
As a result, CloroxPro has been doing yearly evaluations on the HealthyClean Trained Specialist Course since its launch in 2021. Below are some results from last year’s assessment.5 As time goes on, CloroxPro plans to expand to also include Behavior Change and Business Outcomes, so keep an eye on this space for more outcomes.

In conclusion, while most cleaning professionals recognize the importance of training, it is difficult to know which are worth their time. Fortunately, there are well established models like the Kirkpatrick Model that can help.
In this blog, I shared what the Kirkpatrick model is, how it helps with determining the value of a training, and some results from the HealthyClean Certificate Program as an example of how CloroxPro is putting the model to work for their own program.
To learn more about high quality education & training, check out my other blogs:
- Three C’s of Credibility
- Why Instructional Design Matters
- The ABCs of Credentialing
- Help Is Here! Becoming a Successful “Cleaning for Health” Manager
References
1. 2023 CMM In-House/Facility Management Benchmarking Survey Report [Internet]. Cleaning & Maintenance Management. Available from: https://cmmonline.com/articles/2023-cmm-in-house-facility-management-benchmarking-survey-report
2. Kirkpatrick DL. The Four Levels of Evaluation. Evaluating Corporate Training: Models and Issues. 1998; 46:95–112.
3. Kirkpatrick Partners | About Us | Donald L. Kirkpatrick, PhD [Internet]. Kirkpatrick Partners, LLC. Available from: https://www.kirkpatrickpartners.com/about-us/don-kirkpatrick/
4. Standard Practice for Certificate Programs [Internet]. www.astm.org. [cited 2024 Feb 13]. Available from: https://www.astm.org/e2659-18.html
5. Strazdas L and Rops, M. 2023; CloroxPro HealthyClean 2023 Program Evaluation

The epidemiology of Mpox (previously Monkeypox) continues to evolve with a new outbreak being caused by a different “strain”. Since 2022, person-to-person transmission of Mpox has been sustained in a global outbreak, primarily among men who have sex with men (MSM). The stigma associated with this disease has negatively impacted containment of this virus with only 1 in 4 at-risk persons being immunized.1 Mpox continues to circulate in the U.S. and modeling data suggest that if vaccine coverage does not increase, large outbreaks could be expected in the future.2 This would most certainly result in a percentage of these patients being hospitalized with risk of transmission within the hospitals walls.
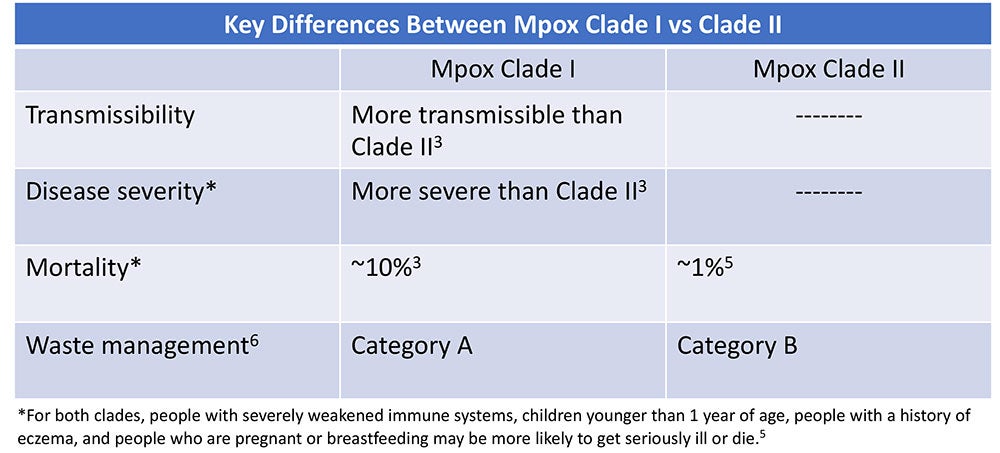
About MPox Clades
Clades are groups of genetically linked viruses. Mpox consists of two clades: Mpox Clade II which causes less severe disease and Mpox Clade I which is more transmissible and causes more severe disease with a mortality rate as high as 10%.3 In early December 2023, the Centers for Disease Control and Prevention (CDC) issued a health advisory of a sexually-transmitted Mpox Clade I outbreak in the Democratic Republic of the Congo (DRC).1 Clinicians were alerted to the possibility of Mpox Clade I in travelers who have been in the DRC. To date, no Mpox Clade I cases have been identified in the U.S., however the World Health Organization (WHO) states there is moderate risk of Mpox Clade I further spreading to worldwide.1,4
What Healthcare Professionals Should Know
The good news is that treatment and prevention measures are the same for both Mpox clades.3 At-risk persons should get vaccinated and should seek medical attention if they have symptoms.3 The virus can be spread from person-to-person from contact with Mpox lesions or respiratory secretions and droplets or from contact with contaminated materials, including soft surfaces like bedding or clothing.3 Because Mpox is spread by direct contact, frequent hand hygiene and routine cleaning and disinfection of environmental surfaces is crucial.3,7 Disinfectants for emerging viral pathogens from the Environmental Protection Agency’s (EPA) List Q should be used.8 The EPA has extended the use for List Q disinfectants for mpox through May 2025.9 Learn more here about navigating List Q as it relates to Mpox.

The risk for occupational transmission of Mpox Clade II to healthcare workers (HCWs) is considered to be low — in fact, only a few cases have been reported.10 However, Mpox Clade I is more transmissible so its plausible that the risk to HCWs may be higher. To protect themselves, HCWs should do the following:
- Adhere to standard and transmission-based precautions, including appropriate PPE.7
- Perform frequent hand hygiene.
- Frequently clean and disinfect high-touch surfaces in mpox patient rooms.
- Avoid dry dusting, sweeping, or vacuuming which can aerosolize the virus.7
- Perform aerosol-generating procedures in a negative pressure room.7
- Never shake or handle soiled linens in a manner that may disperse infectious material.7
- Handle waste in accordance with federal, state, and local regulations. Mpox Clade I is Category A Waste, while Mpox Clade II is Category B Waste.6
The epidemiology of mpox continues to evolve with a substantial increase in the more pathogenic strain of mpox (Clade I) in the DRC.1 Healthcare workers should continue to be vigilant and consider mpox when evaluating the cause of rashes.1 For more information, see our Mpox Pathogen Education Sheet.
References
1. Centers for Disease Control and Prevention (CDC). HAN Alert: Mpox Caused by Human-to-Human Transmission of Monkeypox Virus with Geographic Spread in the Democratic Republic of the Congo; Dec 7, 2023 [Internet]. [cited 2023 Dec 31]. Available from https://emergency.cdc.gov/han/2023/han00501.asp
2. Centers for Disease Control and Prevention (CDC). Mpox Updates for Clinicians, Dec 20, 2023 [Internet]. [cited 2023 Dec 31]. Available from https://www.cdc.gov/nchhstp/dear_colleague/2023/dcl-122023-mpox-updates.html
3. World Health Organization (WHO). Mpox: What we know; Dec 1, 2023 [Internet]. [cited 2023 Dec 31]. Available from https://www.who.int/multi-media/details/mpox-what-we-know
4. World Health Organization (WHO). Multi-country outbreak of mpox; Dec 22, 2023 [Internet]. [cited 2023 Dec 31]. Available from https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-31---22-december-2023
5. Centers for Disease Control and Prevention (CDC). About Mpox [Internet]. [cited 2024 Jan 1]. Available from https://www.cdc.gov/poxvirus/mpox/about/index.html
6. U.S. Department of Transportation. Transporting Infectious Substances Overview [Internet]. [cited 2024 Jan 1]. Available from https://www.phmsa.dot.gov/transporting-infectious-substances/transporting-infectious-substances-overview
7. Centers for Disease Control and Prevention (CDC). Mpox: Infection Prevention and Control of Mpox in Healthcare Settings [Internet]. [cited 2023 Dec 31]. Available from https://www.cdc.gov/poxvirus/mpox/clinicians/infection-control-healthcare.html#anchor_1653508940308
8. US Environmental Protection Agency (EPA). Selected EPA-Registered Disinfectants [Internet]. [cited 2023 Dec 31]. Available from https://www.epa.gov/pesticide-registration/selected-epa-registered-disinfectants
9. US Environmental Protection Agency (EPA). Emerging Viral Pathogen Guidance and Status for Antimicrobial Pesticides [Internet]. [cited 2023 Dec 31]. Available from https://www.epa.gov/pesticide-registration/emerging-viral-pathogen-guidance-and-status-antimicrobial-pesticides
10. Alarcón J, Kim M, Balanji N, Davis A, Mata F, Karan A, et al. Occupational Monkeypox Virus Transmission to Healthcare Worker, California, USA, 2022. Emerg Infect Dis. 2023;29(2):435-437. https://doi.org/10.3201/eid2902.221750
The 21st century world is being impacted by emerging pathogens on an unprecedented scale.¹ Not only are we experiencing diagnostic and treatment challenges that come with emerging pathogens, but there can also be implications for cleaning and disinfecting against a novel pathogen.
Where to start?
It is imperative to select appropriate disinfecting products when dealing with emerging pathogens. But when a pathogen is novel, it is not likely that a disinfectant on the market will have claims against it — at least not right away — and adding new claims to an existing product does not happen overnight. It takes about a year to get new claims approved by the US Environmental Protection Agency (EPA).
So, how do you identify an effective product? The first step is to see if any specific policies, requirements, or resources have been issued in relation to the novel pathogen(s) in question. For example, the EPA's Emerging Viral Pathogen Policy and (e.g., List N, List K, List Q) can be used to find appropriate products (Image 1). It is important to note that the EPA’s Emerging Viral Pathogen policy only applies to viruses. It does not apply to emerging bacteria, fungi, or other pathogens.
Understanding EPA’s List Q
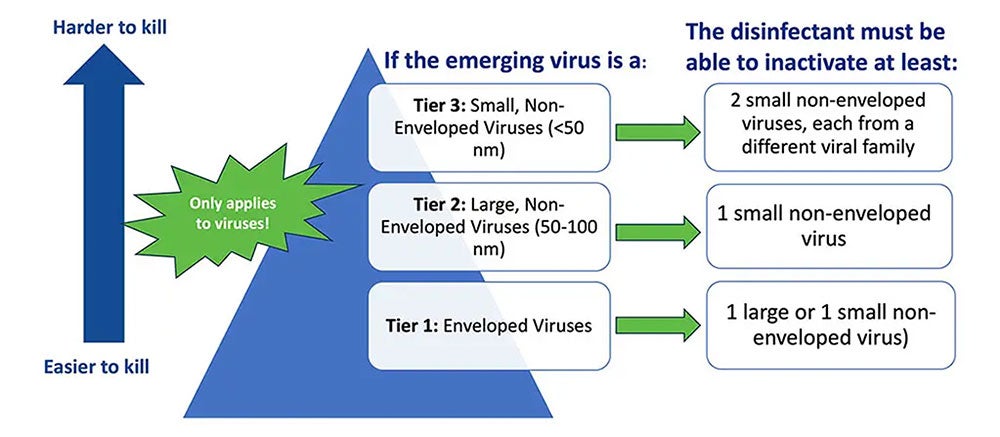
List Q is unique from the other EPA Lists because it does not target a specific pathogen. With this List, the user searches for eligible products for use against an emerging pathogen by selecting the appropriate tier based on virus type.
To navigate List Q, there are a few things you need to know about the emerging pathogen including if it is:
- An enveloped virus (tier 1- the easiest of the 3 types to kill),
- A large non-enveloped virus (tier 2), or a
- A small non-enveloped virus (tier 3 – the hardest of the virus types to kill).
Lastly, it is important to understand that because a given product from List Q meets the criteria for use against one emerging virus (e.g., Mpox), it does not mean this product will be effective against any future emerging viruses. It is designed to be used on a pathogen-by-pathogen basis.2
In situations when guidance like the EPAs Emerging Viral Pathogen policy is not activated, I recommend using a sporicidal, like bleach, because it is broad-spectrum against hard-to-kill pathogens (see CloroxPro “Bleach is B.E.S.T.” blog). Please note — some disinfecting chemistries may be more effective than others against a given pathogen. For example, disinfectants with quaternary ammonium compounds (e.g., “quats”) as the only active ingredient have not been found to be effective against Candida auris.3
Control and contain
Beyond identifying the correct product to use, it is also imperative to identify a process for managing an emerging pathogen. For example, protocols should include the personal protective equipment (PPE) to be worn to enter the room, cleaning frequency, and the appropriate disinfectant to be used. Cleaning and disinfecting protocols may differ by pathogen, so it is also important that staff are educated and trained to safely and effectively clean and disinfect against novel pathogens.
Additional Resources
In addition to the EPA's Emerging Viral Pathogen Policy and the EPA Disinfectant Lists, other organizations have published helpful resources you can use to help you identify the appropriate disinfecting products to use and implement a process for managing an emerging pathogen. The Centers for Disease Control and Prevention (CDC) guidance, the National Emerging Special Pathogens Training and Education Center (NETEC) is another great resource for guidelines. You can also check out the CloroxPro HealthyClean® online learning platform for more training and education opportunities for yourself or your staff.
References
1. Ambat A, Vyas N. Assessment and preparedness against emerging infectious disease among private hospitals in a district of South India. Open Access: Med J Armed Forces India [internet]. 2022;78(1):42-46.
2. US Environmental Protection Agency. Disinfectants for Emerging Viral Pathogens (EVPs): List Q [Internet]. [cited 2023 Aug 14]. Available from https://www.epa.gov/pesticide-registration/disinfectants-emerging-viral-pathogens-evps-list-q
3. Centers for Disease Control and Prevention. Infection Prevention and Control for Candida auris [Internet]. [cited 2023 Aug 14]. Available from https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html

There is a silent public health threat more deadly than COVID-19 killing an estimated 6.2 million people each year across the globe.1 For perspective, COVID-19 killed 6.9 million people globally…but over a 3-year period!2 The threat: antimicrobial resistance (AMR). The challenge with AMR pathogens is that they are not killed by the antimicrobial drugs typically used to treat them.3 AMR is now the third leading cause of death worldwide and is poised to rival the annual all-cancer death toll by the year 2050 with an estimated 10 million deaths.1,4
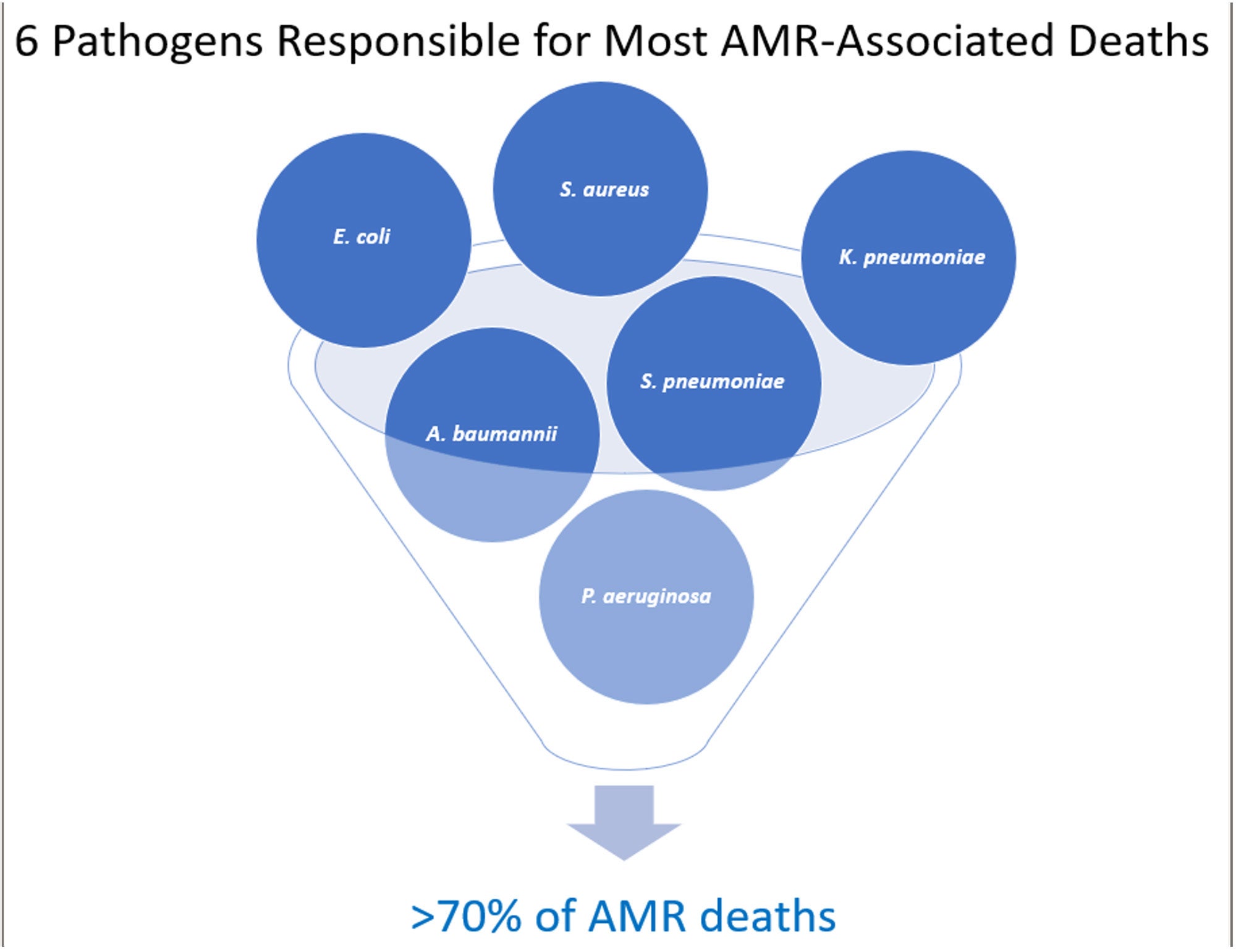
AMR is often referred to as the “Silent Pandemic” because it represents a slow and hidden threat that has the potential to cause widespread health consequences.5 With AMR continuously increasing and fewer new treatments in development, many experts believe that we are not entering, but rather we are living in the post-antimicrobial era.6 Surprisingly, only 6 pathogens are responsible for most of these infections.1
AMR Is Not a U.S. Problem — or Is It?
More than 3 million Americans acquire an AMR infection each year.7 While not in the top 6, Clostridioides difficile (C. diff) is also considered to be an AMR pathogen because it is associated with taking antibiotics. C. diff causes close to 50,000 U.S. deaths annually.8
AMR was an issue before the pandemic, but pandemic-related factors have only intensified the problem including increased use of antibiotics as well as a healthcare system that was stressed to its limits. This made compliance with infection prevention and control measures more challenging8. There were also severe staffing shortages during this time which could have resulted in cutting corners. Replacement staff may not have been adequately trained in proper procedures. The result has been a significant increase in AMR with deaths increasing by 15% in just the first year of the pandemic.8
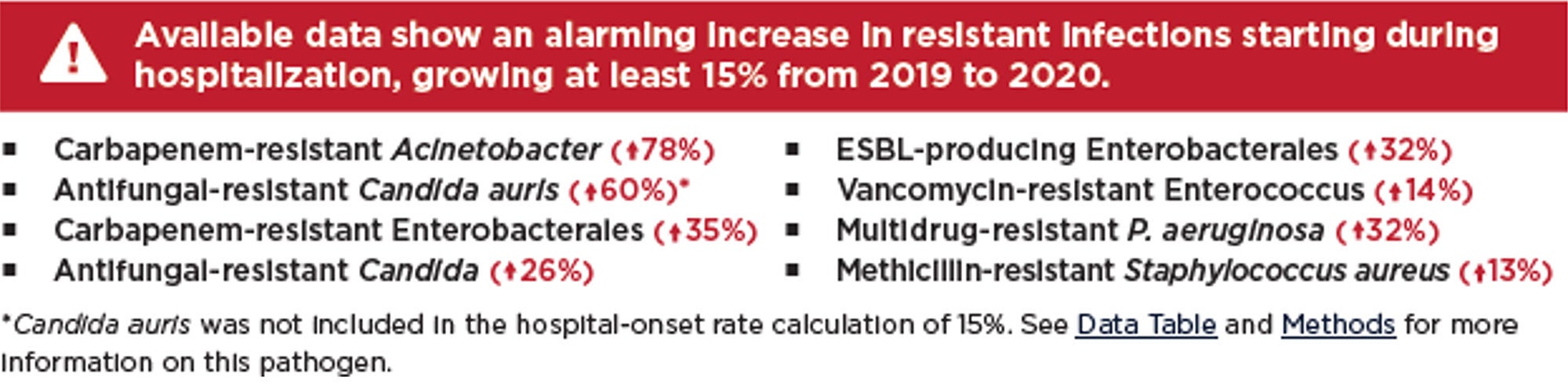
With the COVID-19 public health emergency declared over, our attention has been turned to other emerging and re-emerging microbial threats like Candida auris, Mpox, seasonal respiratory viruses, and even vaccine-preventable diseases. But if the future effectiveness of antimicrobial agents is to be preserved, it is imperative that AMR is given the same focused attention as the afore-mentioned pathogens. Recognized each year in November, World Antimicrobial Resistance Awareness Week (WAAW), is a campaign intended to do just that.9 This blog hopes to help WAAW in raising awareness of AMR and to promote the importance of a sanitary healthcare environment.
Slowing the Rate of AMR
WAAW’s theme this year is “Preventing antimicrobial resistance together.”8 The causes of AMR are complex due to the interconnectivity between people, animals, and the environment (learn more at CDC’s One Health). A multimodal approach, in which everyone plays a role, is crucial to slowing the rate of AMR and preserving antibiotics for future generations.
Key actions that the healthcare community can take to reduce AMR include:
- Perform pathogen surveillance for resistance patterns.
- Establish robust antimicrobial stewardship programs.
- Encourage immunizations of patients and staff.
- Perform frequent hand hygiene.
- Educate and train all healthcare staff on IPC measures to stop AMR spread.
- Ensure effective environmental cleaning and disinfection.
- Ensure cleaning and disinfection of medical equipment between patients.
The Relationship Between AMR and Environmental Cleaning & Disinfection
According to hygiene expert Dr. Nico Mutters, there are 3 reasons that AMR is increasing10:
- Selection due to improper antimicrobial use.
- Transmission resulting from inadequate IPC and hygiene (hands and environmental cleaning).
- Host factors.
Studies show that more than 74% of healthcare surfaces are contaminated with AMR pathogens and that more than 50% of these surfaces that should be cleaned are missed.11,12,13 There is considerable evidence that environmental surface contamination plays a key role in the transmission of many pathogens, particularly those that are AMR. There is sufficient evidence to show that the next patient to be admitted to a room where the prior occupant harbored an AMR pathogen has up to a 3-fold increased risk for acquiring that pathogen.14 This is almost certainly due to inadequate cleaning and disinfection. Environmental cleaning and disinfection breaks the chain of infection by preventing transmission from contaminated environmental surfaces. By preventing infections, the need for antibiotics is reduced, thereby decreasing the risk resistance. A clean and sanitary environment also reduces the direct transmission of AMR pathogens between patients.

To combat AMR, I recommend selecting products that target pathogens of concern for the facility and that meet the users desired properties of the ideal disinfectant. Sporicidal disinfectants (e.g., bleach), with its broad antimicrobial activity, are ideal for combatting AMR. I also recommend using a sporicidal disinfectant for all discharge and terminal room cleans. This helps to ensure the highest level of cleanliness for the next patient admitted to that room.
Conclusion
The rates of AMR have only worsened in recent years and treatment options are becoming more limited. AMR infections come with high consequences including increased lengths of stay, increased cost, and can even result in loss of life. The time for action to reverse this trend is NOW. Learn more here.
References
1. Antimicrobial Resistance Collaborators. Global burden of antimicrobial resistance in 2019: a systematic analysis. Open Access Lancet [Internet]. 2022; DOI: https://doi.org/10.1016/S0140-6736(21)02724-0.
2. Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) [Internet]. [cited 2023 Oct 28]. Available from: https://gisanddata.maps.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6
3. Centers for Disease Control and Prevention. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS) [Internet]. [cited 2023 Nov 02]. Available from: https://www.cdc.gov/narms/resources/glossary.html
4. United Nations. UN News: Reduce Pollution to combat ‘superbugs’ and other antimicrobial resistance [Internet]. [cited 2023 Nov 02]. Available from https://news.un.org/en/story/2023/02/1133227#:~:text=Overall%2C%20nearly%20five%20million%20deaths,globally%20by%20cancer%20in%202020.
5. Google Scholar. Search term “Silent Pandemic” [Internet]. [cited 2023 Oct 29]. Available from: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=silent+pandemic&btnG=
6. European Congress of Clinical Microbiology & Infectious Diseases. Press Release ECCMID 2023, Copenhagen, 15-18 April [Internet]. [cited 2023 Oct 29]. Available from: https://www.eurekalert.org/news-releases/982758
7. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States [Internet]. [cited 2023 Oct 30]. Available from: https://www.cdc.gov/drugresistance/biggest-threats.html
8. Centers for Disease Control and Prevention. 2022 Special Report: COVID-19 U.S. Impact on Antimicrobial Resistance [Internet]. [cited 2023 Oct 30]. Available from: CDC.
9. World Health Organization. World AMR Awareness Week 2023 [Internet]. [cited 2023 Oct 28]. Available from: WHO.
10. Mutters, N. Proceedings from International Conference on Prevention & Control (ICPIC) 2023. Session: Are we prepared for future challenges in infection prevention and control?
11. Carling PC, Bartley JM. Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. AM J Infect Control. 2010;38:S41-50
12. McKinnell J, Miller L, Singh R, Walters D, Peterson E, Huang S. High Prevalence of MDRO Colonization in 28 NHs: An Iceberg Effect. JAMDA. 2020;21(12):1937-1943
13. Cassone M, Wang J, Lansing B, Mantey J, Gibson K, Gontjes K, et al. Proceedings from SHEA 2022. Poster: Diversity and persistence of MRSA and VRE in NHs: Environmental screening and whole-genome sequencing. ASHE. 2022;2:s80.
14. Chemaly R, Simmons S, Dale C, Ghantoji S, Rodriguez M, Gubb J, et al. The role of the healthcare environment in the spread of MDROs: update on current best practices. Ther Adv Infect Dis. 2014;2(3-4), 79-90.

Have you ever wondered what infection prevention and control (IPC) “looks like” in other parts of the world, and how our challenges compare? In the U.S., most Infection Preventionists (IPs) are budgeted to attend at least one conference per year, and international conferences are typically not within the budget. At least, this was the case when I worked in hospitals. After 24 years as an IP, my dream finally came true! I am eternally grateful to Clorox Healthcare for sending me to the International Consortium on Prevention and Infection Control (ICPIC) in Geneva, Switzerland this September. This blog is dedicated to honor Clean Hospitals Day, celebrated each year on October 20th, to raise awareness and foster engagement among healthcare facilities around the world on the important role of environmental hygiene in IPC.
ICPIC is a unique forum for the exchange of knowledge and experience in the prevention of healthcare associated infections (HAIs) and antimicrobial resistance (AMR). It also happens to be the largest IPC conference in Europe. The 1200+ participants, who traveled from across the globe, were stimulated by the high quality of the sessions offered as well as the expertise and reputation of the speakers. Participants came from a variety of disciplines including but not limited to IPs, infectious disease physicians and researchers, public health professionals, academics, and frontline healthcare workers. Speakers included renown researchers and experts from (among others) academia, the Centers for Disease Control (CDC), the Society for Healthcare Epidemiology of America (SHEA), and the World Health Organization (WHO). In case you are wondering, the conference was entirely in English with the provision of simultaneous translation equipment available at every seat.
The excitement at the ICPIC opening ceremonies was palpable and set the tone for the rest of the conference. In her opening remarks, Celine Gardio, Program Director for the Swiss Federal Office for Public Health, provided a call to action to get IPC back on track following the pandemic. Tedros Ghebreyesus, WHO General Director, reminded participants that “without correct IPC, hospitals can become transmitters of infection.” He stated that “hospitals should be places of health, not harm,” yet a sobering statistic remains that globally, most HAIs are AMR. This was a great introduction to the WHO’s new global strategy on IPC, an operational framework using change theory on those actions needed to reduce HAIs, AMR, and infectious disease outbreaks by the year 2030. Dr. Michael Bell, Deputy Director of the U.S. CDCs Division of Healthcare Quality Promotion echoed the concerns of his international colleagues with the growing threats of AMR. I encourage IPs to review the following tools provided by the WHO.
Over the course of the next two-and-a-half days, I attended a plethora of excellent general sessions, keynotes, and symposiums. The over-arching theme of the conference encompassed moving forward in a post-pandemic world. See Image 1 for topics that trended throughout the week.

One trending topic that seemed to be woven throughout the conference sessions, even more so than hand hygiene, was healthcare environmental hygiene. This important topic was finally given the attention it deserves and was emphasized in multiple sessions by the US and international experts alike. Selected experts, including Dr. Didier Pittet (Switzerland), Dr. Alexandra Peters (Switzerland), Dr. Pierre Parneix (France), Dr. David Calfee (US), and Jon Otter, PhD (UK) from the global Clean Hospitals Initiative led a very well-attended symposium dedicated to this topic. The Clean Hospitals Initiative is a network of experts and industry partners (including Clorox Healthcare) working together to make hospitals safer through improved environmental hygiene through academic research, education and training, and raising awareness (learn more here from my recent interview with Dr. Didier Pittet). I truly appreciate how the experts tied a clean environment to clean hands. You can’t have one without the other. I am sure you have all heard me say over the years that our hands are only as clean as the environmental surfaces around us. In a session titled “Are we prepared for future challenges in IPC”, Vanessa Vazquez, a healthcare IP leader (Spain), reminded participants that “infection transmission is not only about our hands”, while another expert panelist, Dr. Moi Lin Ling, Director of Infection Control, Singapore General Hospital, added that “we must pay more attention to environmental hygiene - much like we also do for hand hygiene.” We know that environmental contamination leads to HAIs and that admission to a room previously occupied by a patient with an AMR pathogen, including C. difficile, significantly increases the risk for the next patient admitted to the room to acquiring that pathogen.1,2 In fact, Dr. Alexandra Peters with the Clean Hospitals Initiative and the Hospital University of Geneva (HUG) reported that over the last 20 years, we are seeing a shift towards outbreaks of gram-negative pathogens which are often found in the healthcare environment. Dr. David Weber (United States), in his session on “no-touch room disinfection,”, shared that 20-40% of HAIs are associated with either environmental contamination or cross-contamination. But the good news is that “improving cleaning strategies improves patient outcomes” (Dr. Parneix).
"Our hands are only as clean as the environmental surfaces around us."
– Doe Kley, Infection Prevention Fellow, Clorox Healthcare
To help IPs and environmental services professionals improve their environmental hygiene programs, Clean Hospitals recently released their comprehensive and validated Healthcare Environmental Hygiene Self-Assessment Framework (HEHSAF) – a new tool with the dual purpose of assessing the level of need, but also for improving a facility’s performance by utilizing it as a benchmark for improvement over time. The HEHSAF is based on the Multimodal Improvement Strategy and focuses on both the technical and human elements of healthcare environmental hygiene. This tool, developed by Dr. Pittet and team, follows in the success of a similar tool (“Hand Hygiene Self-Assessment Framework”) developed back in 2010 by this team in collaboration with the WHO. Many IPs reading this blog may be familiar with this tool — I know I employed to improved hand hygiene at my last hospital. I encourage IPs and EVS to work together to complete the HEHSAF which is currently available in 7 languages with more coming.
"Clean care is safer care, a basic patient right"
– Aleksandra Maczynska (Ireland)
To end on a less serious note, I would like to share what I learned in a really awesome session titled “Space: The Final Frontier” presented by Dr. Leonard Mermel, a researcher at Brown University (United States). We know that IPs work in a variety of settings, but did you know that even the National Aeronautics and Space Administration (NASA) employs IPs? If you ever find yourself on Mars, here is what you need to know about life (and health) in a zero-gravity setting:
- Humans become immunosuppressed in space.
- In contrast, microbes grow exuberantly, have enhanced virulence, increased drug-resistance, and a lower infectious dose.
- Chronic viruses (e.g., Epstein Barr Virus, Cytomegalovirus, Varicella) are reactivated.
- When an astronaut coughs or sneezes or flushes the toilet, the droplets remain suspended in the air indefinitely posing unique IPC challenges.
In closing, I give the ICPIC conference a 10 of 10. In response to my own question regarding IPC challenges posed at the opening of this blog, it should not have come as a surprise to learn that IPs across the globe struggle with the same challenges that you and I face every day. Whether you practice in a low-resourced country versus a higher resourced country, perhaps you’ll find it reassuring to know that we all face the same issues in getting staff to consistently comply with IPC practices, including hand hygiene. Pathogens don’t respect state or country borders, but when we work together towards the same goal, we can (and do) make a difference. Because the ICPIC conference was so rich in information, please be on the lookout for a second blog sometime in the near future covering some other great content. I will close with one of my favorite quotes of the week regarding the comparison of healthcare errors to the aviation industry:
"If a pilot crashes, he crashes with his passengers. If a clinician 'crashes,' only the patient is harmed."
– Dr. Andreas Widmer, Swissnoso (Switzerland)
We can and we must improve.

References
1. Weber D, Kanamori H, Rutala W. ‘No touch’ technologies for environmental decontamination: focus on ultraviolet devices and hydrogen peroxide systems. Curr Op Infect Dis. 2016;29 (4).
2. Weber D, Anderson D, Rutala W. The role of the surface environment in HAIs. Curr Opinion in Infect Dis. 2013.

With 90% of companies requiring a return-to-office (RTO) by 2024, the demand for cleaning for health has never been higher.1 In fact, a recent survey of facility executives found that 40% would pay a higher price for "increased infection control procedures."2 With increased awareness of germs, our cleaning staff is under more pressure than ever before. But here's the catch: the turnover rate is soaring, and many are cleaning without proper training.3 This scenario has left our cleaning managers juggling a daunting array of tasks, leading to exhaustion and burnout.
In this blog, I'll shed light on crucial aspects that cleaning for health managers must grasp for success. This includes understanding what cleaning for health is, becoming familiar with best practices, and mastering the art of management that are important with these practices. While I aim to offer valuable information here, the realm of knowledge extends far beyond this scope. If you're eager to dive deeper, I encourage you to explore the wealth of information available in the new ANSI National Accreditation Board-accredited HealthyClean® Trained Manager Certificate Course generously offered for free by CloroxPro. Seize this opportunity today and enhance your expertise and credibility in the world of cleaning for health with this brand-new program! More information is available below.
Decoding Cleaning for Health
Cleaning for health is the process of maintaining and keeping buildings visibly clean and reducing the spread of germs and other unwanted matter.3 To clean for health requires specific approaches, best practices and tools that are focused on removing unwanted matter from a building that has the potential to impact health. While it should be the goal of every cleaning operation, due to its complexity and differences in how it needs to get done, that is not always the case. At the onset, cleaning for health appears to place additional demands on an operation, but in fact, it can be incorporated and even lead to reductions in time and labor when implemented properly.
3 Cleaning for Health Cleaning Essentials for Managers
- Cleaning and disinfecting can help break the chain of infection: One way that germs spread from person to person is through inanimate objects like door handles, light switches, and elevator buttons. When done properly, cleaning can interrupt the spread by killing germs on surfaces so that they cannot spread. Conversely, cleaning improperly can have the opposite effect, by cross-contaminating in the indoor environment.
- Safe techniques help avoid injury: Lifting with your legs and not your back, mopping 2–3 feet in front of you, not overloading your cart, and asking for help if when lifting heavy items are all things that front-line cleaners can do to help stay safe while working.
- Ready-to-use (RTU) products are efficient, effective, and safe: Not having to prepare a product prior to a shift or going back to the supply closet to refill offers an efficiency that is not often considered when purchasing a product. RTU products are always at the right concentration to work as intended. With RTUs, there is no opportunity to under dilute, which can create a more toxic product, or overdilute, which could cause the product to be ineffective. Frontline cleaners prefer to use RTU products because they know they work, and products that work lead to more efficient operations.
3 Cleaning for Health Managing Essentials for Managers
- Communication is king: Whether it be with front line cleaners or building owners, communication is critical to helping the operation run smoothly. Lack of clear communication leads to lack of appreciation and can also lead to confusion and distrust.
- Standardization keeps it simple: Standardization is using the same equipment, tools, materials, products, and processes across an organization. It makes logistics, purchasing, training, and auditing simpler and more efficient. Using the same products across an organization, color coding clothes, and kitting janitor supplies and tools are examples of standardization.
- Training engages and improves outcomes: When employees feel invested in performance improves, buildings are healthier, and turnover declines.
In the face of increased demands and heightened awareness surrounding hygiene, the role of cleaning for health managers has never been more crucial. As frontline cleaners navigate this challenge, the strain on managers is real too. In this blog, I've explored essential elements of cleaning for health, shedding light on what it is, best practices, and how management’s role is essential. While it provides some foundational understanding, the world of expertise in cleaning for health is much broader than this. To further improve your knowledge and skills, I encourage you to take advantage of the new opportunity offered by CloroxPro's new Trained Manager course. More information is available below. Empower yourself today and embrace the path toward becoming a proficient and successful cleaning for health manager. Together, we can elevate our standards and create safer, healthier environments for all!
The CloroxPro HealthyClean Certificate Program offers high-quality and FREE education and training on Cleaning for Health for frontline cleaners & managers, including:
- NEW! An ANSI National Accreditation Board-accredited Trained Manager course on Cleaning for Health and designed for cleaning managers.
- An ANSI National Accreditation Board-accredited Trained Specialist course on Cleaning for Health and designed for front-line cleaners and managers (available in both English & Spanish)
- A 20 min microlearning module on cleaning and disinfecting in healthcare and designed for EVS managers.
- 3 short (5-7 min) microlearning modules addressing some of the most challenging commercial cleaning tasks designed for cleaning managers. These modules each introduce a CloroxPro product that can help.
Become a successful cleaning for health manager by supplementing your current program with ours today!
- To learn more or sign up, visit our website at www.cloroxpro.com/healthyclean
- To learn more about what high-quality education and training is, check out these blogs on finding the right education and training program:
References
1. Hyken S. Nine Out Of 10 Companies Will Require Employees To Return To The Office [Internet]. Forbes. [cited 2023 Oct 16]. Available from: https://www.forbes.com/sites/shephyken/2023/09/24/nine-out-of-10-companies-will-require-employees-to-return-to-the-office/?sh=56fd0ece2baf
2. Results: 2023 Facility Executive Contractor Expectations Survey [Internet]. CleanLink. [cited 2023 Oct 16]. Available from: https://www.cleanlink.com/cp/article/Results-2023-Facility-Executive-Contractor-Expectations-Survey--29867
3. Five Industrial & Institutional Cleaning Trends That Will Define 2023 - Kline & Company [Internet]. klinegroup.com. 2023 [cited 2023 Oct 16]. Available from: https://klinegroup.com/articles/five-industrial-and-institutional-cleaning-trends-that-will-define-2023/
4. Berry MA. Protecting the Built Environment: Cleaning for Health [Internet]. Amazon. Tricomm Twenty First Pr; 1994 [cited 2023 Oct 16]. Available from: https://www.amazon.com/Protecting-Built-Environment-Cleaning-Health/dp/0963571508

It’s 3 AM in the Neonatal Intensive Care Unit (NICU). I am holding my premature 4-pound baby on my chest in the same rocking chair I have sat in for the past several days. It’s dark, quiet and lonely. The days have melted together as my constant focus remains over this tiny human. Suddenly, there is a quiet knock on the door and a worker appears apologizing profusely and explaining he is there to empty the trash and wipe down certain surfaces. He is kind, considerate, sympathetic, and the first human besides the amazing NICU nurses that I have seen in days. I thank him as I try to move all the cords attached to my infant out of his way. He tells me he has been an Environmental Services (EVS) worker for over 20 years and that he is proud to work in the NICU and knows his work is invaluable to protecting these very vulnerable babies. Not knowing my background, he talks to me about how important cleaning and disinfecting is in preventing the spread of pathogens in the hospital. The pride visible on his masked face is evident. Working the night shift, I wonder how lonely he must be and how few people get to fully appreciate all he is doing to protect their children.
EVS workers are in every healthcare facility across the United States. Those outside of healthcare, often incorrectly use the term “housekeeping” and “janitor” to describe these workers, but in healthcare, they are so much more. EVS professionals are highly trained in disinfection practices against contagious pathogens, such as C. difficile and MRSA, and in cleaning complex medical equipment. During the pandemic, their role became even more critical as we were still learning about the SARS-CoV-2 virus and how it was spread. EVS personnel are often underappreciated and not recognized for their shared responsibility to help prevent healthcare-associated infections from spreading on surfaces by prioritizing high-risk areas, and following guidelines for cleaning and disinfection.1,2,3
September 10-16, 2023 is Environmental Services Week #EVSWeek
According to the Association for the Health Care Environment (AHE):
“Environmental Services Week is a time to show appreciation for the dedicated EVS personnel that ensure healthcare facilities across the country are clean, safe places for patients, their families and other staff members. With all the challenges and changes the last few years have brought, these staff members have remained resilient in their efforts to protect others from dangerous pathogens.”
At Clorox, we want to take this week to show our appreciation for the EVS professionals protecting not only countless patients throughout the United States, but also our babies, mothers, fathers, and vulnerable family members. Their tireless efforts keep healthcare facilities safe for all of us!
As Pinnacle Corporate Champion Sponsors of AHE, we have partnered with their team to develop educational tools and training videos to help EVS professionals succeed. These training videos, developed by the CloroxPro Clinical and Scientific Affairs team, highlight critical aspects of cleaning and disinfection in the healthcare environment.

In Research and Development, we are also developing and designing products with EVS in mind. Our ready-to-use wipes allow workers to cover more surfaces with fewer wipes, saving time while still meeting the appropriate contact kill times to ensure dangerous pathogens are not putting patients at risk.
To all the EVS workers out there — we support you and give our thanks for all you do!
References
1. Ni K, Chen B, et al. Knowledge, attitudes, and practices regarding environmental cleaning among environmental service workers in Chinese hospitals. Am J Infect Control [Internet]. 2017 [Cited September 5, 2023]; 45(9):1043‐1045. Available from: https://pubmed.ncbi.nlm.nih.gov/28343703/
2. Bernstein D, Salsgiver E, Simon MS, et al. Knowledge, attitudes, and practices of environmental service workers related to environmental cleaning and healthcare‐associated infections (HAI). Open Forum Infect Dis [Internet]. 2015 [Cited September 5, 2023]; 2(suppl 1): S466. Available from: https://academic.oup.com/ofid/article/2/suppl_1/1711/2634325
3. Ream PS, Tipple AF, Salgado TA, et al. Hospital housekeepers: victims of ineffective hospital waste management. Arch Environ Occup Health [Internet]. 2016 [Cited September 5, 2023]; 71(5):273‐280. Available from: https://pubmed.ncbi.nlm.nih.gov/26359679/
Doe Kley, Infection Prevention Fellow within Clorox Healthcare’s Clinical and Scientific Affairs Team, recently sat down with Professor Didier Pittet of the University of Geneva, Switzerland and Chair of Clean Hospitals to discuss new research, Clean Hospital’s mission and how healthcare professionals can limit the spread of healthcare-associated infections.
With these topics top of mind worldwide, play the video above or keep reading to hear their thoughts.
Please note this is an abridged version of the interview edited for brevity and clarity.
What Is Clean Hospitals?
Doe Kley: Hello, everyone. My name is Doe Kley. I'm an infection prevention fellow with Clorox Healthcare's Clinical and Scientific Affairs Team. I'm a little bit starstruck today because I'm joined by Dr. Didier Pittet to discuss his current work with Clean Hospitals, all the way from Geneva, Switzerland. Welcome, Dr. Pittet. Would you like to introduce yourself and the Clean Hospitals Initiative?
Professor Didier Pittet: Thank you, Doe. It’s a pleasure to be with you today. I'm Professor Didier Pittet from the University of Geneva in Switzerland, and we started the Clean Hospitals initiatives several years ago as a way to partner together to improve patient safety and reduce healthcare associated infections and patient harm in hospitals.
Leveraging the Clean Hands Initiative Framework
Doe Kley: That is awesome. I know that you led the team with the World Health Organization that revamped the hand hygiene guidance. I'm really excited to see what Clean Hospitals accomplishes. I'm curious, was there something that you learned from your hand hygiene work that led you to this next initiative?
Professor Didier Pittet: Absolutely. In fact, the Clean Hospitals Initiative is built on the model that we use for the so-called Clean Hands initiatives or the Clean Hands Save Lives.
First, we did a review of the literature. Is healthcare environmental hygiene important? The answer is yes. You could look at our systematic review that we published a few years ago, that revisited all the studies in the literature, and we paid attention in this review to exclude studies where hand hygiene was promoted. Why? Because we know that if you succeed at promoting hand hygiene, then it would be difficult to monitor the impact of improving healthcare environmental hygiene in reducing infections.
But this systematic review was the first deep research of the Clean Hospital Initiative. [It] was there to help raise awareness about the importance of the role of healthcare environmental hygiene. Then we developed instruments that we already developed for hand hygiene. You probably know the hand hygiene self-assessment framework that we developed in 2009, and we launch globally all over the world.
We have been developing the healthcare environmental hygiene self-assessment framework that we will be launching at the next Clean Hospitals Day worldwide on the 20th of October this year. These are instruments that we used for hand hygiene promotion that we are using in the model of the Clean Hospitals Initiative.
Doe Kley: That's awesome. I'm super excited to get my hands on that self-assessment and share it with some of our customers. I am very familiar with the hand hygiene assessment [and I] used it at my last hospital. It's really incredible to hear about this impactful and inspiring work. Thank you for tackling this important patient safety topic.
The Recipe for Environmental Hygiene
Doe Kley: Beyond what you just shared, I also know that Clean Hospitals champions high-quality products, processes for environmental hygiene. Can you talk just a little bit about this? For example, what does your research show as it relates to the importance of environmental hygiene and patient outcomes or HAIs?
Professor Didier Pittet: If you succeed at implementing a healthcare environmental model program that improves the practices by using not only the right product but the right procedures and sometimes the right people — or training the right people to use the right procedures — you could demonstrate a very significant impact on healthcare associated infections, but also on the transmission of multi resistance, multi resistance and bacteria or multi resistant organism.
This was very revealing of the importance of a multimodal approach for improving healthcare environmental hygiene, something that we know very well in infection control. That's what Clean Hospitals is working on in order to spread this strategy all over the world, using the best partners from all around the world.
Doe Kley: Thank you for that. And I can very much resonate with the multi-modal strategy, especially when you touched on antimicrobial resistant pathogens. We put so much focus and emphasis on antimicrobial stewardship, [it’s] very, very important, but I think we overlook that a clean environment is equally as important. I also think that we are not entering — I think we are in the post antimicrobial era. So even more important now, more than ever before. Thank you for this work and thank you for touching on that.
Ready-to-Use Disinfectants
Doe Kley: Do you have thoughts on ready to use disinfectants in terms of patient safety and [the disinfecting] process?
Professor Didier Pittet: Yeah, of course. The [Clean Hospitals] strategy is a worldwide strategy. So of course, the recommendations may vary from one region of the world to another. Not that bacteria are changing, but that resources are not the same. Sometimes we have only resources that are very simple, but at least we need to make sure that the procedures, the process of cleaning is at the top.
Staff Training
Professor Didier Pittet: That's why we are always adapting the strategy to the level of care and to the resources that are available. Today, we have many approaches to clean the environment. The most important is how are you doing it: with what product, with what technique, and who is doing it. Making sure that people who are performing those processes understand those processes very well. But we need to adapt the technique. The education should be adapted to anybody applying the technique. For us at Clean Hospitals, this is extremely important.
Clean Hospitals Self-Scoring System
Doe Kley: I could not agree more. You know, at Clorox Healthcare, we are huge advocates for compliance, effective [and] efficient cleaning and disinfection. We know it starts with education, training and competency. I think competency is the piece that often gets overlooked. Do you have any thoughts on approach towards achieving competency in our in our cleaning staff?
Professor Didier Pittet: Yes. We have developed a scoring system that will be launched at ICPIC, the Congress in Geneva, but then launched worldwide on Clean Hospitals Day. When you look at the scoring system, it is appreciating. It’s a self-scoring system where you can appreciate the level of your institution. How ready is your hospital? Is your healthcare institution able to have [the] environmental hygiene program adapted to your institution?
In the scoring system, we are looking at the product, we are looking at the process, we are looking at the education. Of course, all of these help us to actually redirect or help the way people can teach, the way people are learning, the way people are implementing. As you know today, infection prevention and control implementation is extremely important.
I know at Clorox you are making sure the implementation of the good product and the good process is a reality and that's extremely important for this. You can use all sorts of techniques, but the most important is to make sure that you are working on the way to implement the best strategies and the best product.
What to Expect at ICPIC
Doe Kley: Perfect. I'm sure that these topics are going to be a focus at this year's International Consortium for Prevention and Infection Control, or the ICPIC conference that you mentioned. Can you share a little bit about Clean Hospitals presence at ICPIC.
Professor Didier Pittet: Clean Hospitals partners [Clorox] will be at the Clean Hospitals booth, where we will show our activities [and] promote our next activities. We will speak about the activities around upcoming Clean Hospitals days.
We will share about our knowledge in the field of healthcare environmental hygiene. There will also be a Clean Hospitals symposium, because at ICPIC we want people to understand the Clean Hospital initiatives and we ask our best international experts from around the world to come and deliver a lecture. We will also have four poster sessions in the field of healthcare and hygiene.
We will have more than 200 posters just in these fields of hand hygiene, healthcare environmental hygiene, from more than 60 countries around the world. Last but not least, Clean Hospitals partners will be there to share and share and share, which is so important for us today in our field.
Doe Kley: I couldn't agree more. It is so exciting to see the increase in activity in the science and the protocols that go into environmental planning and disinfection. I think Clean Hospitals has already started to make a great impact there. Thank you for helping to elevate the hard work. Do you have any final thoughts that you'd like to share with us all Dr. Pettit?
Professor Didier Pittet: We have been saving lives all over the world with the Clean Hands initiative — between 5 and 8 million lives every year around the world — but we know that we can save even more lives. [It’s] so good to have partners like Clorox, among the best partners around the world, being with us in this Clean Hospitals initiative; it's a pleasure and a privilege.
Doe Kley: Thank you. That privilege is all ours. Thank you for inviting us in.


