I often present in multiple forums on environmental cleaning and disinfection in healthcare spaces. Most recently, I had the pleasure of speaking at the APIC 2021 Cleaning, Disinfection, and Sterilization (CDS) conference. My talk was titled “The Next Normal for Cleaning & Disinfection in a Post-Pandemic World.” The session, along with others, was recorded and can be accessed here. This blog shares the questions and answers to some of the more common questions that I receive in the course of my work. Let’s get to it:

Question 1: Can you describe the pros and cons of ATP testing?
Answer:
| Pros | Cons | |
|---|---|---|
| Adenosine Triphosphate (ATP) |
|
|
| Fluorescent marker and black light |
|
|
As ATP and fluorescent markers measure different aspects of the cleaning process, I suggest using a combination of both.
Question 2: Are you aware of any certifications for healthcare cleaning professionals?
Answer:
- The Association for the Healthcare Environment (AHE) offers several, most notable is their Certified Health Care Environmental Services Professional (CHESP). Their newest certification offering is the Certificate in Non-Acute Care Cleaning (CNAAC).
- The Ohio State University (OSU) also has 2 offerings:
- Infection Prevention 101 — this is a free online self-paced course that offers a certificate of completion. Click here to go to the course.
- The second is an online academic undergraduate certificate in healthcare environmental services within the hospitality management program. Click here to learn more about this course.
Question 3: For environmental and high-touch surfaces is it generally acceptable to use a disinfectant wipe for the cleaning step followed with a new wipe for disinfection?
Answer:
It depends. If your product is a 1-step cleaner-disinfectant and the surface is not visibly soiled or the pathogen of concern is not Clostridioides difficile (“C. diff”) or Candida auris, then one wipe can be used to clean and disinfect at the same time. Now if the surface is visibly soiled or C. diff or C. auris are involved, regardless of whether your product is a 1-step or 2-step product, cleaning and disinfection need to be completed in separate steps — and you definitely want to use a new wipe for the second (disinfection) step.
Question 4: Some IFUs for medical devices and equipment state “moist cloth,” but do not state a particular agent. How do you disinfect them?
Answer:
It is a regulatory requirement to follow the manufacturers cleaning and disinfection IFUs. With that said, if the IFUs are not clear or seem inappropriate — for example, a device that comes into contact with a patient skin but the IFUs say clean only with soap and water — there are two actions that can be taken. First, reach out to the manufacturer for clarity. If not provided or still seems inappropriate, submit an FDA Med Watch report. The FDA does not know what they don’t know unless we report these concerns.
Question 5: Can you briefly talk about low-level disinfection versus intermediate-level disinfection?
Answer:
Low-level disinfection is appropriate for the vast majority of your environmental disinfection needs. Intermediate-level disinfection was also required to comply with OSHA’s bloodborne pathogen standard when it was issued in 1992. However, low-level disinfection using products with HIV/HBV claims also comply with the standard.
Question 6: What is the typical contact time for electrostatic disinfection?
Answer:
It depends on the chemistry (disinfectant) being used — be sure to follow the product IFUs for contact time. Now application of the disinfectant by way of electrostatic application is very fast — an entire patient room can be sprayed in a 1–2 minutes depending on the room size.
Question 7: Does electrostatic disinfection technology replace the manual “elbow grease” cleaning?
Answer:
Electrostatics are primarily an adjunct, however, they can be used to disinfect surfaces that are not visibly soiled in between routine cleaning. This Donskey study showed significant reduction in C. diff spores on wheelchairs without a manual cleaning step. Here is one way I think about it, using the wheelchairs again; if these items are getting a thorough cleaning say daily AND they are not visibly soiled, you could spray them between uses using an electrostatic sprayer- I guarantee that it is more disinfection than they would get otherwise in the course of a day. Another example, OR walls can be spot cleaned and then the entire surface disinfected with the electrostatic device - consider the time savings! Regarding the need to clean first. This depends on 2 key things: 1) is the product a one-step cleaner-disinfectant (so many are these days) or is it a 2-step product, and 2) is the surface visibly soiled or is C. diff or C. auris involved? If the surface is not visibly soiled and you have a 1-step cleaner-disinfectant, then a pre-cleaning step is not required. You also have to follow the IFUs — if it says a pre-cleaning step is required, then you must do so. See the EPA's 6 steps for safe and effective disinfectant use. Even they say pre-clean if the directions mention pre-cleaning or if the surface is visibly soiled.
Question 8: Are there any alternatives to using microfiber with quaternary ammonium compounds (“quats”)?
Answer:
Alternatives to using microfiber would be to use a disposable ready-to-use disinfectant wipe. Also, electrostatic application of disinfectant does not have the quat binding issue as no manual wiping required.
I hope you found this Q&A helpful. Keep those questions coming — you can find me on Twitter or LinkedIn. You can also learn more in our Resource and Education Center.
References:
1. Rutala, W. (2019, Aug). Best Practices in Disinfection of Noncritical Surfaces in the Healthcare Setting: A Bundle Approach [PowerPoint Slides]. AHE Exchange Conference.
The COVID-19 pandemic has revealed a number of insights, including the many ways in which schools are important for the health of our children. With the closing of schools to address the spread of COVID-19 infection, a growing body of evidence and expert consensus agree that in-person schooling is critical to the health and development of children. On February 12, 2021, the CDC released updated guidance pushing for reopening schools during COVID-191-2.

What is the basis for the new recommendation that schools can reopen safely?
Many studies have been published recently showing that young children (particularly those in elementary school) are not strong drivers of community transmission of COVID-19. These data suggest that it is possible to reopen schools safely, as long certain mitigation measures are in place to protect teachers, students and school staff. Such measures include physical distancing, wearing masks, improving ventilation, and a focus on effective cleaning and disinfection practices. A recent article in the Journal of the American Medical Association (JAMA) summarized the literature on COVID-19 infection rates in schools3. The authors found that in several schools in the US, school attendance was not associated with increased risk of infection in the school or in the community. Furthermore, in schools with high mask adherence, COVID-19 incidence was lower than in the surrounding community.
What is the new guidance from the CDC?
The new guidance says that schools can reopen safely and provides different strategies to prevent COVID-19 outbreaks based on several factors, including the level of community transmission, the use of COVID-19 testing and screening, and the grade level of the students. For example, elementary schools can be open for some in-person schooling even in communities where COVID-19 transmission is high. By contrast, a high school in a community with moderate COVID-19 transmission may have less in-person schooling.
Overall, the major recommendations for operating schools safely include:
- Universal masking (all students, teachers, staff, and visitors must wear masks)
- Increasing handwashing and hand hygiene practices
- Implementing physical distancing (3 feet between people)
- Improving ventilation in buildings
- Daily cleaning and disinfecting and greater frequency of cleaning and disinfecting of high-touch surfaces
- Incorporating COVID-19 testing and screening
With the above mitigation measures in place, CDC does not recommend that vaccination of teachers, staff, and students be a requirement for reopening. However, CDC does recommend that teachers and staff be given priority for vaccination.
CDC recommends daily cleaning and disinfecting of schools and more frequent disinfecting of high-touch surfaces
As part of a layered mitigation strategy, the CDC has recommended daily cleaning and disinfecting of schools with more frequent disinfecting of high-touch surfaces (such as doorknobs, desk surfaces, sinks and faucets, shared materials, and playground equipment). End-of-day cleaning and disinfecting may still be managed by janitorial or custodial staff members; however, teachers and other school staff will likely need to clean and disinfect high-touch surfaces during the school day. Teachers and other staff should be provided with training, materials, and appropriate personal protective equipment to clean and disinfect safely. General guidance for safe cleaning and disinfecting include:
- Students should not be present during cleaning and disinfecting, and students should not be using cleaning products themselves.
- Choose products for disinfecting that are EPA-registered and on EPA List N. Products on List N have verified claims as a disinfectant and will kill SARS-CoV-2, the virus that causes COVID-19. According to the EPA, disinfectants on List N are also expected to kill all emerging variants of SARS-CoV-2.
- Ensure that staff follow the instructions for use on the product label. Some products may need to remain wet on a surface for up to 10 minutes to be effective.
- Never allow staff to mix cleaning products.
- For surfaces that contact food, have staff do a rinse with potable water after disinfecting if the product label states this is required.
- Staff should wear personal protective equipment as recommended on the product label. This may include gloves, a face mask, a respirator, or eye protection, depending on the type of product.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
REFERENCES
1. Centers for Disease Control and Prevention. (2021). Operational strategy for k-12 schools through phased mitigation. Retrieved February 12, 2021, from https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/operation-strategy.html
2. Centers for Disease Control and Prevention. (2021). Operating schools during covid-19: CDC'S CONSIDERATIONS. Retrieved February 12, 2021, from https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/schools.html
3. Honein, M. A., Barrios, L. C., & Brooks, J. T. (2021). Data and policy to guide opening schools safely to limit the spread of sars-cov-2 infection. JAMA. doi:10.1001/jama.2021.0374
Guidelines from several government and professional organizations lead one to believe that the use of spray disinfectants in healthcare settings is “taboo”. The primary rationale cited for this is concern for the production of aerosols and also for contaminated solutions. In true Infection Preventionist form, I decided to take a closer look at the evidence that informs these guidelines. The bottom line is that I could not find much evidence to support NOT using sprays disinfectants when appropriate, at least nothing current. Let’s take a closer look.
The Evidence
What evidence was used to inform these guidelines? For their recommendations around use of spray disinfectants in healthcare settings, the Centers for Disease Control and Epidemiology (CDC) Guidelines cite four studies ranging from 21-49 years old with little relevance to spray disinfectants.1 As I chased the evidence trail, the studies just got older and older. The most curious finding was the lack of relevance of these studies. The guideline authors are generalizing results from studies that looked at floor care and vacuuming with outdated and faulty equipment, construction activities, use of porous insulation in buildings as a source of pathogens, and prevention of opportunistic infections in stem cell transplant patients to support their stance on not using spray disinfectants.
As for the guidelines from the key professional organizations, most simply cite the CDC’s guidance. Take for example the Association for periOperative Registered Nurses (AORN) Perioperative Practice Guidelines which recommend against the use of spray bottles in the operating room.2 AORN cites the CDC guidelines and states that “sprayed disinfectants produce more aerosols compared to other formats”.2 They also provide the rationale that “if the cleaning solution is contaminated, spraying may provide a route for airborne transmission which may contaminate the surgical wound, sterile supplies, or the sterile field”.2
With today’s pre-diluted, ready-to-use (RTU) sprays and liquids, the chance of contaminated product is virtually zero3. I would also like to call out that we should not be carrying out environmental cleaning tasks when the patient with a surgical wound or sterile supplies are present anyway - regardless of the disinfectant format used!
The Association for the Healthcare Environment (AHE) Practice Guidance provides no rationale or evidence for their recommendation to “apply chemicals using pour spouts, rather than sprayers”.4 Like AORN, AHE recommends “no spraying or misting bottles in the OR as they may aerosolize the disinfectant”. The source for this guidance? AORNs guidelines which point to the CDC Guidance addressed above. As you can see, we are traversing quite the rabbit hole!
Most importantly, I could find no mention on use of spray disinfectants from the Association for Professionals in Infection Control & Epidemiology (APIC) or from the Centers for Medicare & Medicaid (CMS), the latter of which is a regulatory agency.
The basis for non-use of sprays largely centers on the concern of contaminated disinfectants made from concentrate. While this may be possible, the use of RTU sprays and refillable bottles, the risk can be decreased by emphasizing the need to clean and dry spray bottles rather than “topping off”.
Modern Day Sprays
Some of today's manufactures have engineered sprayers that create larger droplets rather than an aerosolized mist which would reduce the risk that spray bottles aerosolize microorganisms or pose an occupational hazard. So if we connect the dots of what we have learned so far, spray disinfectants do have a place for use in healthcare settings.
Think about conducting a risk assessment to determine when and where in your facility that the use of spray bottles might be appropriate. Some examples might include: vacant spaces such as operating rooms between patients or at end of the day, waiting rooms, public restrooms, conference rooms, and public spaces to list a few. When considering a spray disinfectant, include the following in your assessment:
- The conditions in which it would be appropriate to use spray disinfectants.
- Information provided in the Safety Data Sheet (SDS).
- Product instructions for use (IFUs), including personal protective equipment (PPE).
Summary
A reassessment of the evidence from past studies is needed. But in the meantime, we must remember that these are guidelines and not regulations and that healthcare facilities can conduct their own risk assessments and implement the appropriate use of spray disinfectants.
References
- Centers for Disease Control & Prevention (CDC). (2003). Environmental infection Control Guidelines from https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html.
- Association for periOperative Registered Nurses (AORN). (2020). Guidelines for periOperative Practice from https://www.aorn.org/guidelines/about-aorn-guidelines (subscription required).
- Weber, D., Rutala, W., Sickbert-Bennett, E. (2007) Outbreaks Associated with Contaminated Antiseptics and Disinfectants. Antimicrobial Agents and Chemotherapy, p. 4217-4224.
- Association for the Health Care Environment (AHE). (2020). Practice Guidance for Health Care Environmental Cleaning, 3rd edition from https://www.ahe.org/ahe-publications-home (subscription required).
As 2021 rolls around, the U.S. is still in the middle of the COVID-19 pandemic. And just like for most of 2020 we’re likely to continue doing a lot of cleaning and disinfecting in public and private spaces than prior to the pandemic. As a result, people are asking about the effect of more frequent cleaning and disinfecting on common surfaces such as tables, chairs, school desks, countertops, door handles and light switches, and other “high-touch” surfaces.
So what are the issues with extended and frequent use of disinfectants on plastics, metals and other hard, nonporous surfaces, how can they be addressed, and what’s the difference between surface damage, and residue left behind after cleaning and disinfecting?
Residue
Let’s start with the easy one — residue. Most household or commercial disinfectants are water-based. They contain an “active” ingredient which might be a quaternary ammonium chloride, or “quat,” sodium hypochlorite (better known as bleach) or hydrogen peroxide, that kills pathogens. Small amounts of other ingredients are often added; detergents to help with cleaning, special additives to control stability or improve antimicrobial efficacy, and organic solvents to help keep everything in solution and improve performance. After a cleaner or disinfectant applied to a surface has dried, some of these ingredients will be left behind on the surface.

The type, color and texture of the surface material will determine what the residue looks like on the surface. If the disinfectant spreads out evenly on the surface or the surface is textured, the residue may not be easy to see or the surface may appear a little dull. On a smooth surface the disinfectant may form small beads which dry to leave visible spots or circles. If the surface isn’t wiped after application, the residue can build up and appear unsightly. In these situations, you should be able to remove the residue by wiping the surface with a clean damp cloth. You can do this after the contact time has been reached, or periodically — say every few days. You should see no visible change to the surface if the disinfectant hasn’t caused any damage.
Surface damage
Occasionally, after cleaning a surface for an extended period, it may start to look dull or pitted, or you may see hairline cracks on the surface. These are all signs of permanent damage which may have been caused by a frequent use of a cleaning and disinfectant product. While disinfectant manufacturers try to formulate products that won’t cause damage to most hard, nonporous materials, they must balance this with the need for rapid pathogen kill, ease of use, safety and cost. In healthcare settings, where surfaces, medical devices and equipment are frequently cleaned and disinfected, the risk of material damage has been an issue for many years. This issue of “surface compatibility” of the disinfectant is now coming to the forefront in other types of facilities such as schools, colleges and offices which are now being cleaned and disinfected more thoroughly and more frequently.
Surface compatibility is typically described in black and white terms: “This disinfectant is not compatible with this material” for instance. But it’s not that simple. A disinfectant could be used on a surface for many years with no apparent damage to the surface. In other cases, extended use may result in some minor damage — seen as pitting, dulling or tarnishing. This may affect the look of the surface but the structural properties of the material may not be affected. In extreme cases, damage in the form of cracks in plastic materials or corrosion on metals affects the look of the material and indicate that material integrity has been affected. Surface damage not only affects the looks of the material but can also make it harder to clean and disinfect a surface properly.
What you can do to reduce the risk of surface damage?
When disinfectants are used regularly, it’s important to balance the benefits of their efficacy against pathogens versus the risk of potential damage to materials and surfaces. Fortunately, users can take several steps to reduce the risk of surface damage.
- Look at the equipment manufacturer’s cleaning and care guidelines for how to clean and disinfect surfaces. These often include guidance on what types of products can be used and how. If there are no guidelines, contact the equipment manufacturer to discuss care and maintenance.
- Disinfectant manufacturers are increasingly issuing guidance on compatibility of their products with common hard surface materials. Look at their websites or contact them to see what products are compatible with different types of surface materials.
- Just as with residue, wiping with a clean damp cloth to remove excess disinfectant reduces the risk of damage. Depending on the surface material and disinfectant, wiping may need to take place after each cleaning; in other situations, removal every few days may be enough.
As you continue to work hard to provide your customers with healthier and safer environments, factors including the type of surface materials being cleaned, the importance of surfaces looking clean as well as disinfected, and the risk of not disinfecting versus potential damage will all need to be considered. The good news is that there are a few steps you can take to eliminate residue and reduce the potential for surface damage, while keeping the facility environment clean, disinfected and safe.
This post is part of our "Ask The Pros" blog series for which our internal panel of experts address the latest questions from industry professionals. This month's query,
"It seems like we have a new disinfectant product every week and it's hard to keep up with how and where to use the products. What strategies do you recommend for as little disruption as possible to our current process?"
Introduction
The year 2020 has certainly been a challenging one thus far. The COVID-19 pandemic has created supply challenges in this country that we could not have anticipated that range from U.S. Mint coin shortages to personal protective equipment (PPE) for healthcare workers. The disinfectant manufacturing industry has also been challenged to keep up with an extraordinary increase in demand. While production facilities are operating 24/7, supply continues to fall short of demand due to production capacity and raw material shortages. As a result, many healthcare facilities are having to adjust and adapt to new disinfectants products, whether that means different formats, applications, or actives. While this may be frustrating, healthcare teams are resilient and they know how to triage. In response to disinfectant shortages, changes in product and potentially in protocols, we will apply the methodology of triage to the use of disinfectant products in formats that may differ from our usual product. A plan of action can then be developed for the appropriate use of the environmental disinfectants available.
Risk Assessment
The first step when an issue is identified is to assess the risks involved and the potential consequences. In this case, the issue is that our usual product(s) may be temporarily unavailable, or in limited supply. I recommend downloading and adapting a risk assessment tool from the Centers for Disease Prevention and Control (CDC). Considerations with the risk assessment are:
- The patient population
- Location within the facility where the disinfectant will be used
- The staff using the product
- The disinfectant chemistry and format
Once the risk assessment has been completed, the next step is to formulate a plan to mitigate and determine what, if any, safeguards should be put into place. Your plan should include goals and objectives to tackle high-risk issues. Note that your plan should also address how you will swiftly communicate the change in product and educate staff as the new products come in to your facility. Your vendors may be able to help!
Product Prioritization
Much like the CDC has recommended a strategy to prioritize the use of PPE to preserve supply, consider doing something similar in regards to disinfectants. I am not proposing cleaning less frequently, but rather to prioritize which products will be used where and by whom. If you have a limited supply of disinfectant wipes, but you also have some spray bottles of disinfectant, consider prioritizing disinfectant wipes for critical departments, or equipment. For example, you wouldn’t want to use the spray disinfectant in the ICU where you have patients on a ventilator, so this would be a department in critical need of disinfectant wipes. Another example might be to take the large format disinfectant wipes that Environmental Services (EVS) often use and distribute them to nursing staff for use on the units. EVS is more accustomed and likely more equipped to utilize different formats of disinfectants such as spray disinfectants, dilutable chemistries and microfibers so consider reserving these formats for them.
If you need help getting started with how to prioritize your products, check out this flow chart (also pictured below) and associated blank and completed risk assessments for reference.
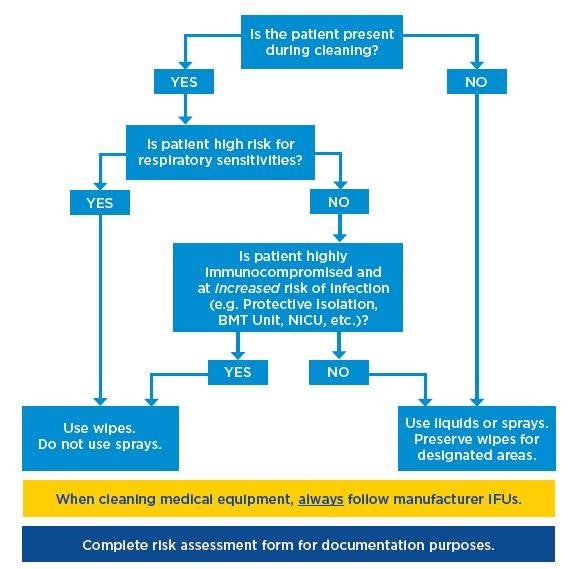
Instructions for Use
Through all of this, it is imperative that the product instructions for use (IFU’s) are reviewed, staff is educated on the IFU’s, and compliance is monitored and enforced by leadership. Healthcare-grade disinfectants registered by the U.S. Environmental Protection Agency (EPA) undergo stringent testing requirements in order to prove their efficacy and safety. For the best results, users should follow the product IFU’s.
For example, Clorox Healthcare® Fuzion, a next-generation sporicidal bleach disinfectant, has an engineered dual-chambered nozzle that combines the active ingredients at the point of dispensing (or spraying). While highly efficacious, this product is most effective when applied directly to the surface from the bottle itself. If concerned about using sprays but that is all that is available, a better approach would be to consider where use of sprays might be more appropriate, such as in public or common areas after-hours. Finally, until this pandemic is behind us, be sure you are selecting products approved as being effective against SARS-CoV-2, the virus responsible for COVID-19 disease. You can find these products on the EPA’s List N.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
Posts for the Ask The Pros blog series are published every other month. Please submit your cleaning and disinfecting questions to AskThePros@clorox.com for consideration to be addressed in a future edition.