In the past year or so, I’ve read about a number of outbreaks of carbapenem-resistant Acinetobacter baumanii (commonly abbreviated to CRAB) that highlight the threat from this particular antibiotic resistant pathogen. Before describing those in more detail, a little about CRAB.
Like bacteria in the Enterobacteriaceae family, A. baumannii is a gram-negative bacterium that can also develop resistance to carbapenem antibiotics. It is part of the Moraxellaceae family and is the most important species causing human infections within that family. The most common resistance genes found in A. baumannii include those that code for New Delhi metallo-beta-lactamase (NDM) and oxacillinase (OXA) enzymes, both of which chemically degrade carbapenem antibiotics. The high levels of resistance mean that the infections caused by CRAB in the bloodstream, lungs, wounds, and urinary tract increasingly require the use of last-line antibiotics such as colistin, polymyxin B and tigecycline.
The first reports of CRAB infections appeared in the early 1990’s but reports have increased steadily since then. Around 800 articles have been published since 1999, and outbreaks have been reported globally.
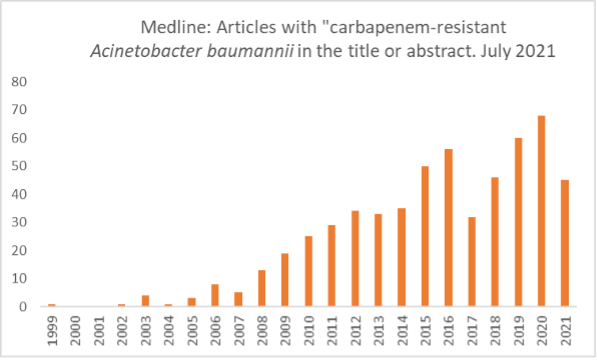
The serious global threat of CRAB
In the CDC’s first Antibiotic Resistance Threats report issued in 2013, multi-drug resistant A. baumanii is listed as a “Serious Threat.”1 Carbapenem resistance is not specifically mentioned. In the 2019 report, CRAB is specifically listed as an “Urgent Threat,” the highest threat level.2 This report estimates that in 2017, there were 8,500 cases in the U.S., resulting in 700 deaths and a significant cost to the health system of $281 million. The one piece of good news is that rates have declined by 25% since 2011. That said, WHO’s June 2021 Global Antimicrobial Resistance and Use Surveillance System (GLASS) report “depicts a dire scenario” noting that a median of 66% of A. baumannii causing bloodstream infections are carbapenem-resistant.3
The challenges of controlling and preventing and controlling CRAB infections
In the first half of 2020, New Jersey, an outbreak at a hospital in New Jersey highlighted the importance of consistently implementing comprehensive infection prevention and control (IPC) measures, especially if patients are intubated and ventilated.4 In the outbreak, 34 patients were infected or colonized with meropenem-resistant A. baumanii infections, 80% of which occurred during the facility’s surge in COVID-19. Of these patients, 25 were intubated and mechanically ventilated and ultimately, 20 with infections were identified. Due to personnel and equipment shortages, space constraints and the high number of critically ill patients admitted during the COVID-19 surge, this hospital understandably had to adopt alternative mitigation measures. As a result, intentional and unintentional changes in IPC measures may have contributed to the outbreak.
A regional outbreak that occurred in May 2021 in California highlights how the infection can rapidly spread between facilities.5 Of 52 NDM-CRAB cases, 43 cases were reported in a single county across multiple facilities including acute care hospitals, two skilled nursing facilities, and a long-term acute care hospital. A further 17 probable NDM-CRAB cases were epidemiologically linked to these cases or facilities. Genome sequencing of 17 isolates pointed to a common source of exposure. This outbreak emphasizes the need for active surveillance including screening new patients being transferred from facilities experiencing outbreaks and placing them on contact precautions while awaiting results.
Finally, an outbreak at a hospital in Israel shows how A. baumanii can persist in the environment.6 Three wards were terminally cleaned with bleach and UV before becoming wards for COVID-19 patients only. Two weeks after reopening, five cases of CRAB infection or colonization were identified, all belonging to the same meropenem-resistant clonal lineage. Three were acquired in a single ward, which prior to COVID-19 had been used to cohort CRAB patients. Epidemiological investigation revealed that the ward’s medication room had not been terminally cleaned and was identified as the source of CRAB. The hospital implemented several measures which stopped the outbreak. The ward in question was closed to new admissions, terminal cleaning repeated, and the medication room closed permanently. CRAB patients were cohorted in a single unit and staff were not permitted to care for CRAB-negative patients until they had left the cohort area and removed all PPE (which included a disposable gown over COVID-19 PPE coveralls.
Preventing transmission of CRAB and other multi-drug resistant organisms
These three outbreak reports paint a picture of the seriousness of CRAB and why the CDC has declared it an “Urgent Threat.” Although in the U.S., cases were steadily decreasing prior to the pandemic, we won’t know how the pandemic has impacted CRAB and other MDRO rates until more data is analyzed and released. In the meantime, these five actions can help to help prevent transmission of CRAB and other MDROs:
- Conduct active surveillance and screen new patients from outbreak facilities
- Test for CRAB and MRDOs through public health labs or the CDC’s AR Lab Network AR Lab Network
- Implement contact precautions along with standard environmental and personal infection control measures
- Communicate a patient’s MDRO status to receiving facilities
- Report carbapenem-resistant organisms and other MDROs to local and state health departments
One final point to note is that if a disinfectant has an EPA-approved claim against A. baumanii, it will most likely not be carbapenem-resistant strain. However, these disinfectants should still be effective against CRAB as drug resistance is not expected to change a bacterium’s susceptibility to disinfectants.
1. Antibiotic Resistance Threats in the United States, 2013. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed July 19, 2021
2. Antibiotic Resistance Threats in the United States, 2019. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf Accessed July 19, 2021
3. Global antimicrobial resistance and use surveillance system (GLASS) report 2021. Geneva: World Health Organization; 2021. License: CC BY-NC-SA 3.0 IGO.
4. Perez S et al. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions — New Jersey, February–July 2020. MMWR / December 4, 2020 / Vol. 69 / No. 48
5. Regional Outbreak of Highly Drug-resistant Carbapenemase-producing Acinetobacter baumannii May 2021. California Department of Public Health. http://publichealth.lacounty.gov/acd/docs/HighlyDrugResistantCarbapenemaseProducingAcinetobacter_baumannii.pdf Accessed July 19, 2021
6. Gottesman T et al. An outbreak of carbapenem-resistant Acinetobacter baumannii in a COVID-19 dedicated hospital. Infection Prevention in Practice. 2021 Mar; 3(1): 100113.
COVID-19 infections caused by the delta variant are surging in much of the United States and globally, just as countries are continuing to open and get back to some level of normal. In the United States, the end of the summer will mark the return to school for millions of kids. Similarly, many businesses are considering how or whether to have workers return to offices and if so, on what schedule. And all over the country, restaurants, movie theaters, concert venues and other entertainment venues are continuing to welcome back customers. As they do so, they continue to implement a range of infection prevention measures recommended by the U.S. Centers for Disease Control and Prevention (CDC) which include among others, promoting vaccination, improving ventilation, regular hand hygiene, wearing of masks, cleaning and disinfecting, COVID-19 testing, and case investigation and contact tracing of employees.
CDC guidance on cleaning and disinfecting non-healthcare facilities has been updated periodically to reflect the current state of evidence, but the general principles and practices have remained the same.1 Although the risk of COVID-19 transmission from surfaces is low, it’s important to remember that other disease-causing bacteria and viruses can also be spread this way. Consequently, regular cleaning and disinfection is important to help keep your facility users healthy.
The most common questions asked about cleaning and disinfecting facilities to help prevent COVID-19 are how often and when. Based on current CDC guidance, cleaning with products containing soap and detergents can decrease the risk of infection from surfaces. Disinfection with an EPA-registered List N disinfectant may further reduce the risk of pathogens. If a COVID-19-positive person has been in the facility within the previous 24 hours, then the facility should be cleaned and disinfected. It’s worth remembering that the EPA expects that all List N disinfectants will kill COVID-19 virus variants, including the Delta variant, which is currently responsible for most of the infections in the United States.
However, there are other situations where more regular cleaning and disinfection may be necessary:
- In high-traffic areas in shared spaces where surfaces could be touched regularly by multiple people. These criteria could apply to many school settings.
- If COVID-19 transmission rates in the community are high. With the current Delta variant surge, this may apply to many parts of the country. In the CDC’s COVID-19 Integrated County View tracker, you can find the level of community transmission for any county in the U.S., classified as low, moderate, substantial and high.2
- If there are low vaccination rates in your community. In the same COVID-19 tracker, you can find out the vaccination rates in any county in the U.S.
- If the wearing of masks by unvaccinated people is low, or hand hygiene is infrequently practiced.
- If your facility is frequently used or occupied by people at a higher risk for severe illness, such as older people, those with chronic health conditions, or those who have long experienced systemic health and social inequities.
This guidance can help facility managers assess the risks of transmission from surfaces and develop appropriate cleaning and disinfecting plans and protocols.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
1. U.S. Centers for Disease Control and Prevention. Cleaning and Disinfecting Your Facility. Every Day and When Someone is Sick. Updated June 15, 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html Accessed August 4, 2021.
2. The County Tracker is part of the CDC’s COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home Accessed August 4, 2021.
In the last few months, it’s emerged that a number of variants of SARS-CoV-2, the virus that causes COVID-19, are circulating globally. Variants arise through a process called mutation where the virus undergoes changes to its genetic structure. The new variants can have characteristics that are different from the original virus. You may see the terms “variant” and “strain” used interchangeably, and in this context, they mean the same thing.
Currently there are three variants of note: one that emerged in the UK in September 2020 and has now been detected in the US, one from South Africa that was first reported in October 2020, and one from Nigeria that emerged at the end of 2020. The UK and South Africa variants appear to spread more rapidly than the original SARS-CoV-2 virus, but do not appear to cause more severe disease and can still be detected by currently available viral tests.
In January 2021, the United States Environmental Protection Agency (EPA) issued a statement saying that it expects disinfectants on List N Disinfectants for Coronavirus (COVID-19) to kill all strains of SARS-CoV-2. This is to be expected based on the structure of viruses. Let’s take a look and see why.
Virus classification
Viruses can be split into three classes. Enveloped viruses, large non-enveloped viruses, and small non-enveloped viruses. An enveloped virus is surrounded by a fatty layer which breaks apart very easily making the virus very easy to kill with disinfectants. Non-enveloped viruses have a tough outer coating making them much harder to kill with disinfectants.
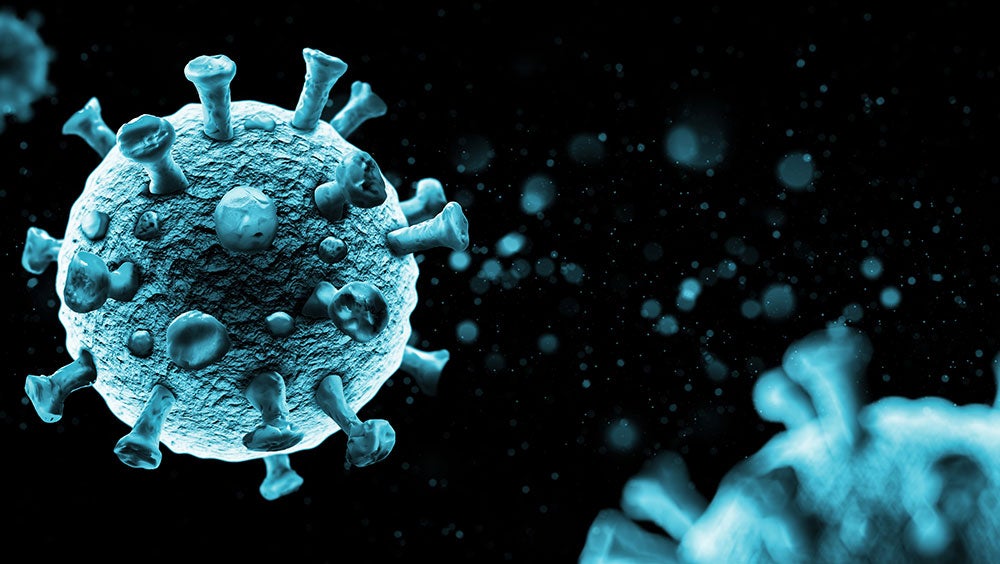
SARS-CoV-2 is an enveloped virus but also contains protein “spikes” that stick through the fatty outer layer, creating a “crown”-like structure — one that you’re no doubt familiar with.
The key point is this: the variant resulting from a mutation in an enveloped virus is still an enveloped virus with the easy to penetrate fatty layer, and therefore equally susceptible to disinfectants as the original. The mutation may slightly change the make-up or characteristics of individual parts, such as the protein spikes, but it does not change the physical structure of the virus. This scientific rationale is the basis for the EPA’s determination that viruses on List N are expected to kill all strains, or variants, of SARS-CoV-2.
Disinfectant antiviral efficacy in practice
In practice, we can see the fact that different strains or variants of viruses are equally easy to kill when we look at kill claims (or contact times) for influenza A viruses on product labels. There are many variants of this virus including H5N1, H1N1, H3N2. However, when the microorganism claims on a product label include more than one variant of this virus, the kill time (or contact time) is always the same for all variants. The EPA recognized this in 2009 when there was an outbreak of a new strain of influenza A, H1N1. At that time, the EPA ruled that any disinfectant that killed a known strain of influenza A would also be effective against the new strain.
Many disinfectants can kill SARS-CoV-2 with contact times ranging from 15 seconds to 2 minutes. As EPA suggests, we can expect that List N disinfectants will be effective against all variants of SARS-CoV-2 including the current ones from the UK, South Africa and Nigeria.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
As 2021 rolls around, the U.S. is still in the middle of the COVID-19 pandemic. And just like for most of 2020 we’re likely to continue doing a lot of cleaning and disinfecting in public and private spaces than prior to the pandemic. As a result, people are asking about the effect of more frequent cleaning and disinfecting on common surfaces such as tables, chairs, school desks, countertops, door handles and light switches, and other “high-touch” surfaces.
So what are the issues with extended and frequent use of disinfectants on plastics, metals and other hard, nonporous surfaces, how can they be addressed, and what’s the difference between surface damage, and residue left behind after cleaning and disinfecting?
Residue
Let’s start with the easy one — residue. Most household or commercial disinfectants are water-based. They contain an “active” ingredient which might be a quaternary ammonium chloride, or “quat,” sodium hypochlorite (better known as bleach) or hydrogen peroxide, that kills pathogens. Small amounts of other ingredients are often added; detergents to help with cleaning, special additives to control stability or improve antimicrobial efficacy, and organic solvents to help keep everything in solution and improve performance. After a cleaner or disinfectant applied to a surface has dried, some of these ingredients will be left behind on the surface.

The type, color and texture of the surface material will determine what the residue looks like on the surface. If the disinfectant spreads out evenly on the surface or the surface is textured, the residue may not be easy to see or the surface may appear a little dull. On a smooth surface the disinfectant may form small beads which dry to leave visible spots or circles. If the surface isn’t wiped after application, the residue can build up and appear unsightly. In these situations, you should be able to remove the residue by wiping the surface with a clean damp cloth. You can do this after the contact time has been reached, or periodically — say every few days. You should see no visible change to the surface if the disinfectant hasn’t caused any damage.
Surface damage
Occasionally, after cleaning a surface for an extended period, it may start to look dull or pitted, or you may see hairline cracks on the surface. These are all signs of permanent damage which may have been caused by a frequent use of a cleaning and disinfectant product. While disinfectant manufacturers try to formulate products that won’t cause damage to most hard, nonporous materials, they must balance this with the need for rapid pathogen kill, ease of use, safety and cost. In healthcare settings, where surfaces, medical devices and equipment are frequently cleaned and disinfected, the risk of material damage has been an issue for many years. This issue of “surface compatibility” of the disinfectant is now coming to the forefront in other types of facilities such as schools, colleges and offices which are now being cleaned and disinfected more thoroughly and more frequently.
Surface compatibility is typically described in black and white terms: “This disinfectant is not compatible with this material” for instance. But it’s not that simple. A disinfectant could be used on a surface for many years with no apparent damage to the surface. In other cases, extended use may result in some minor damage — seen as pitting, dulling or tarnishing. This may affect the look of the surface but the structural properties of the material may not be affected. In extreme cases, damage in the form of cracks in plastic materials or corrosion on metals affects the look of the material and indicate that material integrity has been affected. Surface damage not only affects the looks of the material but can also make it harder to clean and disinfect a surface properly.
What you can do to reduce the risk of surface damage?
When disinfectants are used regularly, it’s important to balance the benefits of their efficacy against pathogens versus the risk of potential damage to materials and surfaces. Fortunately, users can take several steps to reduce the risk of surface damage.
- Look at the equipment manufacturer’s cleaning and care guidelines for how to clean and disinfect surfaces. These often include guidance on what types of products can be used and how. If there are no guidelines, contact the equipment manufacturer to discuss care and maintenance.
- Disinfectant manufacturers are increasingly issuing guidance on compatibility of their products with common hard surface materials. Look at their websites or contact them to see what products are compatible with different types of surface materials.
- Just as with residue, wiping with a clean damp cloth to remove excess disinfectant reduces the risk of damage. Depending on the surface material and disinfectant, wiping may need to take place after each cleaning; in other situations, removal every few days may be enough.
As you continue to work hard to provide your customers with healthier and safer environments, factors including the type of surface materials being cleaned, the importance of surfaces looking clean as well as disinfected, and the risk of not disinfecting versus potential damage will all need to be considered. The good news is that there are a few steps you can take to eliminate residue and reduce the potential for surface damage, while keeping the facility environment clean, disinfected and safe.
This is the first of a series of historical blog posts, each of which will cover the life and work of infection control pioneers or celebrate key related events. This post features Sir John Pringle whose original ideas laid the foundation for aspects of infection control that we take for granted today.
On the first day of January 1750, the Philosophical Transactions of the Royal Society published three remarkable articles. The first, titled “Some experiments on substances resisting putrefaction” was followed by two others, both of which described additional experiments on the topic. The articles are notable because they describe the first scientific study on these substances which the author called “antiseptics”, and they represent one of the earliest uses of the word “antiseptic”, a term which some have attributed to the author.
That author was Sir John Pringle. Readers of Infection Control and Hospital Epidemiology (ICHE) may well be familiar with him, as his portrait has graced the front cover of every volume in 2020. He sits at a desk facing the unknown artist, looking as if he’s been interrupted while reading a manuscript, and holds a walking stick in his left hand. He wears the typical dress of a gentleman of his time; black breeches falling just below the knee, white stockings, a long, dark coat, and a fashionable silvery-gray wig. The journal gives a brief description of his achievements but there’s much more to Sir John, physician to the army and the Royal Family, President of the Royal Society, and one of the pre-eminent physicians of his day.

Sir John, it turns out, was far ahead of his time. His pioneering work on antiseptics, combined with his work on his constant aim of “preventing infection, the common and fatal consequence of a large and crowded hospital” perhaps entitles him to be considered not just a founding father of military medicine, but as a pioneer of public health and infection prevention practices.
His experiences on the battlefields during the War of the Austrian Succession in the 1740’s appears to have shaped much of his thinking around transmission and prevention of infectious diseases. His most important work, Observations on the Diseases of the Army, published in 1750 gave, for the first time, a scientific account of the epidemiology, pathogenesis and prevention of hospital cross-infections. He observed that military hospitals with their poor ventilation, unsanitary conditions and overcrowding were a chief cause of sickness and death, citing for instance, the spread of dysentery between patients in the same wards through infectious straw probably used as bedding.
He also recognized that outbreaks of jail fever and hospital fever were the same disease – typhus, reaching this conclusion after observing that deserters with jail fever transmitted the disease to English troops, who then became the source of hospital fever outbreaks. His advice for prevention of flea and lice-spread typhus is as sound today as it was then; burn the clothes of prisoners and executed criminals and give new clothes to prisoners and wash them from time to time. Furthermore, these observations led him to describe interventions to moderate or prevent the contagion including dispersing the sick, preserving pure air in the wards, reducing overcrowding, along with other hygienic measures to combat sepsis.
That idea that pure air could help prevent the spread of infection became apparent in October 1750. After an outbreak of jail fever in Newgate Prison and the law courts of the Old Bailey (the latter had taken the life of the Lord Mayor of London), Sir John joined a committee to investigate. It had a clear objective:
“to inquire into the best means for procuring in Newgate such a purity of air, as might prevent the rise of those infectious distempers, which not only had been destructive to the prisoners themselves, but dangerous to others, who had any communication with them”.
A Royal Society publication of 1753 describes the investigation and the solution which was the installation of a windmill-powered ventilator invented by the leading ventilation expert of the time, the Reverend Dr. Stephen Hales. This device “sweetened” the air in the building by drawing out the foul air from the lower stories to the upper. After installation, deaths in the prison decreased from eight a week to about two a month, but not until seven of the workmen installing the device died of the fever. Two hundred and seventy years later, the importance of good ventilation to help prevent the spread of COVID-19 is becoming very clear.
But back to antiseptics, and a connection to modern day disinfectants. The Royal Society publications describe in meticulous detail, Sir John’s controlled experiments on a range of exotic-sounding substances. His method was simple – add the substance to a small piece of meat in water, cap the tube and keep it warm, then observe whether the meat putrefied or not. Substances that prevented or stopped putrefaction he called “antiseptic”. What the substances were doing was killing or preventing the growth of bacteria that would cause putrefaction.
At this point, it’s worth remembering that although van Leeuwenhoek had observed bacteria through his microscope in 1676, they were virtually ignored for over 100 years and not well known at the time of Sir John’s experiments. In fact, nowhere in the three publications does the word “bacteria” appear.
Of the countless exotic-sounding and common substances tested, he found that alkalis as Spirit of Hartshorn (aqueous ammonia solution derived from the horns of a male red deer) and salammoniac (ammonium chloride) and acids such as vinegar (acetic acid) and lemon juice (citric acid) were effective antiseptics. This is perhaps not surprising to the modern reader with a basic knowledge of antimicrobial mechanism of action. For these substances, or derivatives of them form the basis of many disinfectants today; quaternary ammonium chlorides are essentially ammonium salts; acetic acid when combined with hydrogen peroxide forms peracetic acid, an especially powerful antimicrobial agent. And citric acid is finding its place as an EPA-approved Safer Choice active antimicrobial ingredient.
We have a lot to thank Sir John Pringle for; basic infection prevention measures for hospitals, the idea of fresh air and good hygiene, fundamental research into antiseptics. Well-thought of in his time – there is even a memorial to him in Westminster Abbey – his contributions seem to have been forgotten over time. Thanks to ICHE for bringing him to life in 2020. I’m eagerly waiting to see who’s on the front cover in 2021.
Originally published by CleanLink.
About a year ago in this post, I asked the question “What can the U.S. learn from Australia’s 2019 Flu Season?”. One year later, and although a lot has changed since then, I’m asking myself the same question again.
Last year, Australia’s flu season came two months earlier than normal and peaked around June. Early on, most of the cases were caused by influenza A, but as the season wore on, the proportion of cases caused by influenza B increased. The 2019 U.S. flu season also came a little early, but was characterized by an initial spike of influenza B which then receded before influenza A hit.
Of course, we’re living in an entirely different world to the one 12 months ago. In the U.S., there have been some warnings and concern that the country may get hit with a double whammy of COVID-19 and influenza during this year’s flu season. However, as we look at the current data coming out of Australia, which is in the middle of winter and therefore its influenza season, provides an interesting perspective and may contain learnings for the U.S.
In Australia, lockdown began in late March, a little before influenza cases generally start to pick up. Many areas of the country eased restrictions toward the end of May and into June, but some went back into lockdown in July in response to regional surges in cases. This is typically peak influenza season in Australia. For much of the lockdown period, wearing a mask was only recommended for those caring for a COVID-19 patient, but recently, the health department has recommended wearing masks when community transmission is occurring and when physical distancing is difficult. At least one state, Victoria, has made masks mandatory. Additionally, as in most countries around the world, schools and universities have been closed and public gatherings prohibited.
Like COVID-19, influenza is a respiratory illness and is spread in similar ways, mostly by droplet transmission. Consequently, with these precautions in place, it’s interesting to learn that influenza cases in Australia are markedly down compared to 2019, a particularly bad year. Numbers from Australia’s National Notifiable Diseases Surveillance System show that in the first seven months of 2020, Australia reported 21,000 cases of influenza compared with around 214,000 for the same period of time in 2019. Deaths for that same period are also down, from 486 in 2019 to 36 in 2020. These are huge reductions.
"I think if we could get this sort of effect every year, we'd be very happy," said Professor Ian Barr, deputy director of the World Health Organization Collaborating Centre for Reference and Research on Influenza, to the Australian Broadcasting Corporation.
This one bright spot in an otherwise difficult year has been welcomed amongst Australian medical professionals. The lower rate of community transmission of influenza is perhaps not surprising; the precautionary measures such as social distancing being taken for COVID-19 also help prevent the spread of influenza and have likely saved hundreds of lives. Additionally, the shutting down of schools and aggressively closing borders are likely to be major contributors. A 30% increase in the number of influenza vaccinations administered is also thought to have helped Australia keep its influenza cases lower.
So the lessons seem clear: social distancing, the wearing of masks in public places, the closure of public spaces that are generally hotbeds for the transmission of influenza, border closures, and high vaccination rates can all help prevent the spread of COVID-19 AND influenza. And these results aren’t just limited to Australia. Other countries in the southern hemisphere such as Argentina, Chile, and South Africa are also reporting large decreases in influenza cases as well, thanks in part to the implementation of COVID-19 prevention measures.
The U.S. may have already seen the benefits of these measures earlier this year. Speaking to the U.S. edition of the The Guardian, Dr. Richard Kennedy of the Mayo Clinic’s vaccine research lab said that shutdown measures “shaved four to six weeks” off the U.S.’s 2019-2020 flu season.
Unfortunately, as we’ve seen, implementation of such preventative measures varies widely across the country, and border closures have not been as aggressively implemented as in Australia. Meanwhile, flu vaccination rates are also low – according to the Centers for Disease Prevention and Control (CDC), flu vaccination rates hover at around 50% or less each year.
With flu season approaching, it seems reasonable to assume that measures to help prevent the spread of COVID-19 can also help to prevent the spread of influenza. Aggressively adhering to these measures gives the U.S. the chance of avoiding the “double whammy” of a winter of COVID-19 AND influenza, something that may once again place an enormous burden on hospitals and communities nationwide.
Updated July 31 to reflect additional updates from the EPA regarding List N.
As a follow-up to Richard’s earlier blog post, here’s a short update on the current status of EPA’s List N, Disinfectants for Use Against SARS-CoV-2 (COVID-19).

The EPA’s emerging viral pathogens guidance for COVID-19 has been in effect for a little over five months. The guidance outlines criteria that disinfectants must meet to enable the EPA to approve their use against SARS-CoV-2, the virus that causes COVID-19. To qualify, disinfectants must demonstrate efficacy against a harder to kill virus such as a large or small non-enveloped virus. The emerging viral pathogen guidance was designed exactly for situations like the COVID-19 pandemic, when the emerging virus causing the disease isn’t immediately available to laboratories to conduct disinfectant microefficacy testing to support label claims.
However, for a few months now, the SARS-CoV-2 virus has been available for testing, and as you’d expect, multiple companies are having their disinfectants tested for efficacy. In July, the EPA announced that fifteen products had become the first to demonstrate efficacy against the virus following testing according to EPA-approved methods. These products can add a SARS-CoV-2 efficacy claim to their federal Master Labels. More will certainly follow. For all products, the efficacy data must still be reviewed by the California Department of Pesticide Regulations (DPR), and individual states will need to accept new labeling before the claim can be added to product labels.
What This Means for Disinfectant Selection
Let’s take a look at what this actually means. For several months now, the EPA has provided a list of disinfectants that can be used against this coronavirus. This is List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19). The number of products on List N has increased each time the EPA has included a new criterion that a disinfectant can meet to be included. The EPA expects products on List N to kill SARS-CoV-2 because they meet one of four criteria:
- The product has an emerging viral pathogens claim, meaning that the products have demonstrated efficacy against a virus that is harder to kill than SARS-CoV-2 (COVID-19).
- The product has demonstrated efficacy against SARS-CoV-2 (COVID-19).
- The product has demonstrated efficacy against a harder-to-kill pathogen than SARS-CoV-2 (COVID-19), specifically norovirus, Mycobacterium boyis | Mycobacterium tuberculosis.
- The product has demonstrated efficacy against another type of human coronavirus similar to SARS-CoV-2 (COVID-19).
As of July 28, 2020, there are 468 products on the list. The majority of disinfectants on List N — 308 of them — are included because they have an emerging viral pathogens claim. An additional 102 disinfectants are included because they have an efficacy claim against a human coronavirus similar to SARS-CoV-2. With the availability of SARS-CoV-2 for microefficacy testing, we finally see products — currently 15 — that are included on the list because they have been shown to effectively kill the SARS-CoV-2 following testing according to an EPA-approved method. The remainder of products are effective against human coronavirus or a harder-to-kill pathogen than SARS-CoV-2.
What’s important to note is that EPA considers ALL of the products on List N to be effective and approved for use against SARS-CoV-2, even though only 15 have actually demonstrated efficacy against the SARS-CoV-2 virus in microefficacy tests.
Why You Should Still Use List N
Given the potential for the COVID-19 pandemic to be long-lasting, it’s understood that List N will continue to be updated and remain as a resource, even as more products are tested against SARS-CoV-2. The emerging viral pathogens guidance is based on a well-established and thoroughly researched hierarchy of virus susceptibility to disinfectants. SARS-CoV-2 is an enveloped virus, making it an easy virus to kill. With the proof of disinfectant efficacy being generated through EPA-approved methods, combined with the results of recent published studies, it’s clear that many household disinfectants as well as disinfectants designed for use in healthcare and other professional settings should be able to kill the virus on hard non-porous surfaces. This supports the rationale behind the EPA’s emerging viral pathogens guidance, and criteria for inclusion on List N. Those looking for a disinfectant to use against SARS-CoV-2 can, and should, select any of the disinfectants on List N and be reassured that the product they are using will align with EPA and CDC guidance for cleaning and disinfecting against SARS-CoV-2.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
Originally published by Infection Control Today. For more information on SARS-CoV-2, click here.
With cases of COVID-19 now confirmed in the United States, the question inevitably being asked by industry professionals is “What disinfectants can I use against this virus?”
Fortunately, regulatory and public health agencies in the U.S. provide clear guidance on what products may be used in outbreaks of emerging pathogens.
In the U.S., the U.S. Environmental Protection Agency provides emerging viral pathogen guidance on the criteria disinfectants need to meet to be considered effective against an emerging pathogen.

The efficacy criteria are based on the ease with which the three types of viruses — enveloped, large non-enveloped and small non-enveloped viruses — are inactivated by disinfectants. The general idea of the EPA’s policy is that in order for a disinfectant to be considered effective against an emerging pathogen, it must demonstrate efficacy — that is, have an EPA-approved claim – against viruses that are harder to kill than the emerging pathogen.
SARS-CoV-2, the virus responsible for the COVID-19 outbreak, is an enveloped virus and therefore the easiest to kill of the three types of viruses. This means the efficacy criteria for a disinfectant is as follows:
- A disinfectant must be EPA-approved as a hospital/healthcare or broad spectrum disinfectant
- The disinfectant must carry an EPA-approved claim for at least one small or one large non-enveloped virus
- Lastly, the EPA-approved master label must contain emerging pathogen “terms of registration” language describing what emerging pathogen claims the manufacturer can make. Typically, this takes the form of language such as:
Emerging Viral Pathogen Claims
Allowable and subject to the terms described in Agency guidance dated August 19, 2016, “Guidance to Registrants: Process for Making Claims Against Emerging Viral Pathogens not on EPA-Registered Disinfectant Labels
This product qualifies for emerging pathogen claims against:
- Enveloped viruses
- Large non-enveloped viruses
- Small non-enveloped viruses
If the disinfectant satisfies all three criteria, the manufacturer can communicate this information via technical literature distributed exclusively to healthcare facilities, physicians, nurses and public health officials, "1-800" consumer information services, social media sites and company websites, using specific permitted language. The required language must be written in one of the following formats:
[Product name] has demonstrated effectiveness against viruses similar to [name of emerging virus] on hard, [porous and/or nonporous surfaces]. Therefore, [product name] can be used against [name of emerging virus] when used in accordance with the directions for use against [name of supporting virus(es)] on [hard, porous/nonporous surfaces]. Refer to the [CDC or OIE] website at [pathogen-specific website address] for additional information.
[Name of illness/outbreak] is caused by [name of emerging virus]. [Product name] kills similar viruses and therefore can be used against [name of supporting virus(es)] on [hard, porous/non-porous surfaces]. Refer to the [CDC or OIE] website at [website address] for additional information.
For example, for the current COVID-19 outbreak, a statement using the first example of permitted language for a disinfectant with a claim against Rhinovirus (a small, non-enveloped virus) would read:
Disinfectant X has demonstrated effectiveness against viruses similar to SARS-CoV-2 (formerly 2019-nCoV) on hard, nonporous surfaces. Therefore, Disinfectant X can be used against SARS-CoV-2 (formerly 2019-nCoV) when used in accordance with the directions for use against Rhinovirus on hard, nonporous surfaces. Refer to the CDC or OIE website at https://www.cdc.gov/coronavirus/index.html for additional information.
So now that you know the requirements, what steps can you take to find an effective disinfectant? It’s always best to contact the manufacturer or check its website for disinfectants the manufacturer has determined to have EPA-approved emerging viral pathogens claims. Many manufacturers have issued guidance including lists of eligible products. While looking at manufacturer websites, it is important to look at the specific language used. Claims should not read as “kills SARS-CoV-2” or “effective against SARS-CoV-2,” as this new pathogen is not yet available for EPA testing. Given the new name of SARS-CoV-2 was confirmed on February 12, 2020, some manufacturers may still be using the previous name for the virus, 2019-nCoV.
With the SARS-CoV-2 outbreak continuing, and with COVID-19 cases in the U.S. increasing, albeit slowly, it’s critical that the right information on disinfection selection is provided to healthcare, cleaning and janitorial industry professionals. By following trusted guidance from national regulatory and public health agencies, facilities can help ensure they are best prepared for this as well as any future outbreaks of viral pathogens.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
The Joint Commission adds three new FAQs related to cleaning and disinfecting medical equipment in its Interpretation Standards
Situation
The Joint Commission recently added three new FAQs to its Standards Interpretation webpage. The three new FAQs relate to cleaning and disinfecting medical equipment and should be very helpful to healthcare facilities that need to regularly clean and disinfect medical devices and equipment to ensure patient safety.

Background Information on The Joint Commission
Joint Commission standard IC.02.02.01 requires that the hospital reduces risk of infection associated with medical equipment, devices, and supplies. It is expected that a facility will follow instructions for use (IFUs) for cleaning and disinfection standards provided by medical device and equipment manufacturers.
However, in many situations, manufacturers’ IFUs for cleaning and disinfection are unclear, insufficient to enable correct cleaning and disinfection necessary to reduce the risk from infection, or do not permit/include the use of cleaning and disinfecting products that a particular facility currently uses.
The Joint Commission FAQs provide clarification regarding the responsibilities of manufacturers and facility leadership, as it relates to provision and availability of IFUs for cleaning and disinfecting medical devices and equipment. They also provide guidance on what actions to take when currently used cleaning and disinfecting products may not be compatible with manufacturers’ device and equipment cleaning and care IFUs.
What Each of the FAQs Say
This FAQ confirms the FDA requirement that manufacturers of medical devices and equipment provide instructions on how to clean, disinfect and if necessary, sterilize their products. The IFUs should include the steps required, the level of disinfection (low/intermediate-, high-level or sterilization), frequency and which products are compatible.
- The FAQ also confirms that cleaning, disinfection and sterilization products should contain IFUs, which must be followed. For low-level disinfection IFUs, these will primarily apply to correct dilution, contact time and, if applicable, application method.
FAQ #2: Manufacturers Instructions for Use — Responsibility for Ensuring Availability
This FAQ clarifies that facility leadership is responsible for ensuring that IFUs for medical devices and equipment are available for use by staff, and that compliance with IFUs is an integral part of facility policies, protocols, staff education, and training and competency assessments. It also describes actions that leadership can take to support this, which include:
- Using IFUs provided by equipment and device manufacturers and ensuring that staff are trained on how and where to access electronic copies, for example, subscribing to web-based resources that maintain IFUs
- Training staff on how to read and implement IFUs, incorporating them into policies, protocols and/or standard operating procedures
- Alerting staff when the disinfectant used in the hospital cannot be used to clean equipment or devices
This FAQ describes what organizations should do when they “identify a conflict amongst the IFUs for different equipment and products.” Although the term “conflict” is not described, interpretations include a situation where a disinfectant the facility wants to use is not compatible with the medical device or equipment IFU, or a specific disinfectant product is not listed. The FAQ provides actions to take, and illustrates these actions in descriptions of two scenarios:
- Contacting the device/equipment manufacturer’s technical services
- Contact the manufacturer of the “alternative” product to see if they can provide additional information on compatibility – including biological, chemical and functional compatibility (Note: Clorox interprets the “alternative” product as the disinfectant the facility is using)
- Identifying the risks, strategies to mitigate risks and implanting those strategies in situations where clear compatibility information cannot be obtained
- Considering the implications of their decision on liability, warranty and long-term life of the medical device or equipment
Recommendations for Cleaning & Disinfection
The FAQ clarifies the position of The Joint Commission with regards to cleaning and disinfection of medical devices and equipment. Inclusion of this FAQ in the Standards Interpretation section suggests that the development and implementation of cleaning and disinfecting standards and procedures for medical devices and equipment will be a focus of The Joint Commission in the future.
This clarification of responsibilities and guidance on how to balance the benefits of cleaning and disinfection against the risk of potential damage to equipment acknowledges the fact that this has been a challenging part of infection prevention protocols for many years.
To avoid a survey finding, Clorox Healthcare suggests several steps in line with The Joint Commission’s FAQs on cleaning and disinfection of medical devices and equipment:
- Facility leadership should carefully read these FAQs and consider how to implement the recommendations in their facilities.
- If a medical device or equipment IFU states that a Clorox Healthcare product used in the facility is not approved, we encourage facilities to first contact device and equipment manufacturer technical services department to ask for more information about compatibility.
- Consult the Clorox Healthcare Surface Compatibility hub for high-level guidance on compatibility of disinfectants with common healthcare materials and resources to help determine how best to clean and disinfect medical devices and equipment.
- Form collaborative teams to assess the benefits of cleaning and disinfection against the risks of potential equipment damage. Develop protocols that include steps to mitigate the risk of damage, such as wiping down surfaces with a clean, damp cloth after the disinfectant contact time has been reached.
- Consider alerting Biomedical Engineering that more frequent preventative maintenance on certain equipment may be needed.
Note: The FAQ are included in manuals for the different types of healthcare settings; the first two FAQs are included in the “Infection Prevention & Control” chapter while all three appear in the “Leadership” chapter.
How do you solve a problem like the sink drain? So goes the line in possibly the Sound of Music’s most well-known song. Well, perhaps not, but there’s no denying that sinks and drains and the nasty microorganisms that inhabit them are a growing problem in healthcare.

For a number of years now, researchers all over the world have been investigating sinks and drains as a potential source of hospital-acquired infection (HAI)-causing microorganisms, especially carbapenem-resistant Enterobacteriaceae (CRE). CRE infections are of particular concern because of their ability to transfer their resistance genes from one bacterial species to another, say from carbapenem- resistant Klebsiella pneumoniae to Escherichia coli, and they're difficulty to treat.
In 2000, a paper in Current Opinions in Microbiology posed the question “Is the emergence of carbapenemase a problem waiting to happen?” Almost 20 years on, the problem is here. Reports that suspected sinks and drains could be the source of CRE that caused HAIs began to emerge in 2003. In 2017 a review, The Hospital Water Environment as a Reservoir for CRE Causing Hospital-Acquired Infections, included 17 reports that identified sinks as a potential source of microorganisms, including CRE, that caused HAI outbreaks, often in the ICU.
More reports have appeared in the literature since then. Recently, two investigations of outbreaks in ICU in China and Israel employed molecular methods to confirm that ICU sinks were indeed the cause of CRE infection outbreaks.
So how does CRE get from the drains to patients?
Some excellent work from the United States has increased our understanding of this. It turns out that bacteria can spread along common pipes connecting sinks and colonize the p-trap. Once there, they form a biofilm which can grow upwards to reach the sink strainer at a rate of up to an inch a day if fed with nutrients such as juice or coffee. Then, as the U.S. study and one from the United Kingdom show, water from the faucet directly hitting the sink strainer can splash on to surrounding counters and the floor up to three feet away from the sink, bringing with it the bacteria that have contaminated the sink strainer. If splashing does occur, patient care items left around the sink can become contaminated, increasing the chance of transmission of pathogens to patients. It’s the CRE and other pathogens isolated from sink drains and transmitted via this splashing that are suspected as the source of hospital-acquired infections.
What have hospitals done to tackle sinks as a source of CRE?
Not surprisingly, hospitals have tried a range of interventions to stop outbreaks, with varying degrees of success, and the 2017 review covers these. They include:
- Replacing the entire contaminated sink units or replacing the downpipes and p-traps (both costly)
- Regularly pouring disinfectants down the sink
- Using a device that heats and subjects the downpipe to ultrasound to kill and remove the biofilm.
Some interventions resulted in the end of the outbreak but didn’t fully eliminate CRE from the environment and the potential of a CRE infection occurring. Others were not successful at eliminating the outbreak at all.
What interventions are being researched?
Pouring disinfectants such as bleach, hydrogen peroxide and acetic down sink drains significantly decreases bioburden but regrowth happens within a few days. Blocking the drain and allowing the disinfectant to sit in the p-trap and downpipe is more successful, with regrowth taking longer. A simple device that covers the sink strainer and held in place with three suction cups is able to successfully prevent water from the faucet hitting the sink strainer and then splashing on to sink surrounds. A much more complex intervention consisting of a sink bowl that limits water dispersion and a mechanism that enables the faucet to dispense disinfecting ozonized water is effective at decreasing Pseudomonas aeruginosa and Candida auris contamination but is clearly a more costly intervention. Research in this area is likely to continue.
What does CDC suggest?
The Centers for Disease Control and Prevention's (CDC) guidance document, Reduce Risk from Water: From Plumbing to Patients, includes a section on how to reduce the risks of transmission of MDROs from sinks, drains and plumbing. Many recommendations have their origins in the reports in the literature and include:
- Regularly cleaning and disinfecting surfaces near drains and around sinks
- Avoiding placing patient care items on counters next to sinks
- Not placing sinks next to medication preparation areas or separating them with splash barriers
- Preventing faucets from discharging directly above sink strainers, ensuring that water flows are in line with FGI Guidelines
- Installing sinks that reduce the possibility of splashing
- Not discarding patient waste or sources of nutrients such as beverages down sinks
The CDC guidelines stop short of recommending pouring disinfectants down sinks on a regular basis, most likely because no one antimicrobial ingredient has been found to be better than others, and ideal concentrations to use are not known.
It seems clear then that there’s no silver bullet that will completely eliminate the risk of pathogen, and particularly CRE transmission from sinks. Instead, like with many infection prevention solutions, a bundle of small interventions — behavioral, such as better housekeeping around sinks; practical, such as regular cleaning and disinfection; or design-related, such as replacing sinks or putting diverting water from the faucets — will likely be required to solve a problem like the sink drain.