Strategies for Using Unfamiliar Disinfecting Product Formats During COVID-19
This post is part of our “Ask The Pros” blog series for which our internal panel of experts address the latest questions from industry professionals. This month’s query,
“It seems like we have a new disinfectant product every week and it’s hard to keep up with how and where to use the products. What strategies do you recommend for as little disruption as possible to our current process?”
Introduction
The year 2020 has certainly been a challenging one thus far. The COVID-19 pandemic has created supply challenges in this country that we could not have anticipated that range from U.S. Mint coin shortages to personal protective equipment (PPE) for healthcare workers. The disinfectant manufacturing industry has also been challenged to keep up with an extraordinary increase in demand. While production facilities are operating 24/7, supply continues to fall short of demand due to production capacity and raw material shortages. As a result, many healthcare facilities are having to adjust and adapt to new disinfectants products, whether that means different formats, applications, or actives. While this may be frustrating, healthcare teams are resilient and they know how to triage. In response to disinfectant shortages, changes in product and potentially in protocols, we will apply the methodology of triage to the use of disinfectant products in formats that may differ from our usual product. A plan of action can then be developed for the appropriate use of the environmental disinfectants available.
Risk Assessment
The first step when an issue is identified is to assess the risks involved and the potential consequences. In this case, the issue is that our usual product(s) may be temporarily unavailable, or in limited supply. I recommend downloading and adapting a risk assessment tool from the Centers for Disease Prevention and Control (CDC). Considerations with the risk assessment are:
- The patient population
- Location within the facility where the disinfectant will be used
- The staff using the product
- The disinfectant chemistry and format
Once the risk assessment has been completed, the next step is to formulate a plan to mitigate and determine what, if any, safeguards should be put into place. Your plan should include goals and objectives to tackle high-risk issues. Note that your plan should also address how you will swiftly communicate the change in product and educate staff as the new products come in to your facility. Your vendors may be able to help!
Product Prioritization
Much like the CDC has recommended a strategy to prioritize the use of PPE to preserve supply, consider doing something similar in regards to disinfectants. I am not proposing cleaning less frequently, but rather to prioritize which products will be used where and by whom. If you have a limited supply of disinfectant wipes, but you also have some spray bottles of disinfectant, consider prioritizing disinfectant wipes for critical departments, or equipment. For example, you wouldn’t want to use the spray disinfectant in the ICU where you have patients on a ventilator, so this would be a department in critical need of disinfectant wipes. Another example might be to take the large format disinfectant wipes that Environmental Services (EVS) often use and distribute them to nursing staff for use on the units. EVS is more accustomed and likely more equipped to utilize different formats of disinfectants such as spray disinfectants, dilutable chemistries and microfibers so consider reserving these formats for them.
If you need help getting started with how to prioritize your products, check out this flow chart (also pictured below) and associated blank and completed risk assessments for reference.
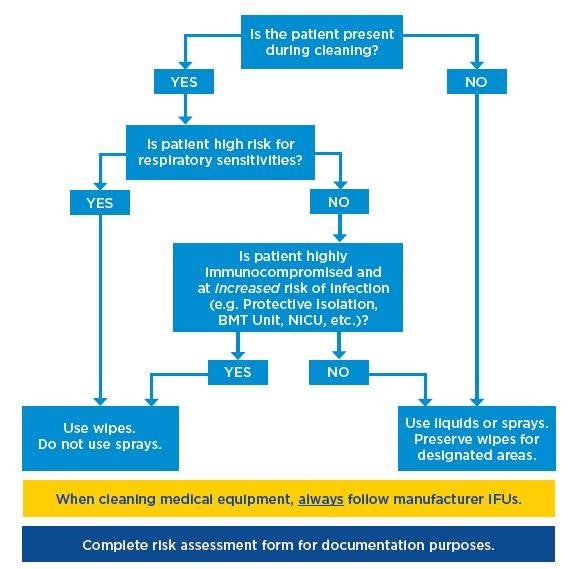
Instructions for Use
Through all of this, it is imperative that the product instructions for use (IFU’s) are reviewed, staff is educated on the IFU’s, and compliance is monitored and enforced by leadership. Healthcare-grade disinfectants registered by the U.S. Environmental Protection Agency (EPA) undergo stringent testing requirements in order to prove their efficacy and safety. For the best results, users should follow the product IFU’s.
For example, Clorox Healthcare® Fuzion, a next-generation sporicidal bleach disinfectant, has an engineered dual-chambered nozzle that combines the active ingredients at the point of dispensing (or spraying). While highly efficacious, this product is most effective when applied directly to the surface from the bottle itself. If concerned about using sprays but that is all that is available, a better approach would be to consider where use of sprays might be more appropriate, such as in public or common areas after-hours. Finally, until this pandemic is behind us, be sure you are selecting products approved as being effective against SARS-CoV-2, the virus responsible for COVID-19 disease. You can find these products on the EPA’s List N.
For the latest information on COVID-19 and variants, visit our CloroxPro COVID-19 Hub.
Posts for the Ask The Pros blog series are published every other month. Please submit your cleaning and disinfecting questions to AskThePros@clorox.com for consideration to be addressed in a future edition.


